Accelerometer 5 G (uses 3 inputs) for MRI
Extend Discovery into the MRI
Advanced technology for MRI-based research
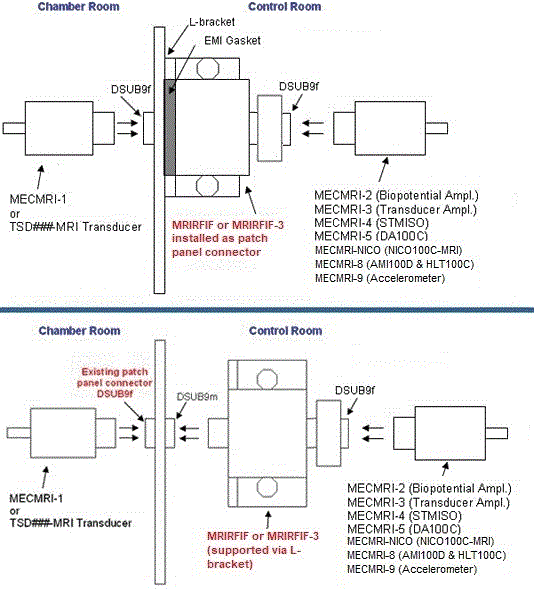
This Tri-Axial Accelerometer high level output transducer (±5 G) is optimal for measuring accelerations when performing slow movements, subtle gestures, or tapping responses and can be used as a feedback transducer for subjects in the MRI. The transducer provides three outputs, simultaneously measuring acceleration along the X-, Y- and Z-axes; ships with a 3-axis interface cable (MECMRI-9 to AMI100D or HLT100C), filter set (MRIRFIF), and two Velcro straps (10 cm and 33 cm).
The frequency response extends from DC to 500 Hz. The accelerometer is extremely accurate and can easily be calibrated by simply changing its orientation in three-dimensional space, so that gravity (G=1) acts only upon the desired axis.
The pliable and unobtrusive design conforms readily to body contours—strap the accelerometer on finger, wrist, toe, or foot.
MRI Use: Conditional to 7T
Condition: Use with provided MECMRI-9 cable and MRIRFIF filter. Conductive parts of transducer are electrically and thermally isolated from subject. (See Specifications for components.)
See More...




Stay Connected