Event Markers | Correcting Automated Placement
Why aren’t event marks always placed correctly by automated analysis routines?
Outliers in graph data—such as noise or other signal artifact—may result in the unwanted or inaccurate placement of event marks during automated analysis routines that use Find Cycle and have peak tracking enabled.
- Examples of analyses using the Find Cycle algorithm include Hemodynamics operations, e.g., Arterial Blood Pressure (ABP); sample setup shown here:
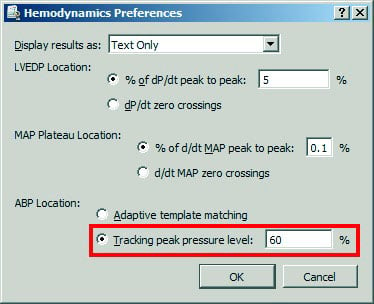
Any unusual area in the data that contains a major jump can throw off the Find Cycle algorithm tracking the threshold and skew the results. Should this occur, this workaround using some basic AcqKnowledge software tools will prove helpful for extracting statistics from relevant data while excluding noise or other unwanted artifacts. These artifacts will be replaced by inputting values into the graph. Ultimately, the values inputted into the graph must fall within the lowest and highest points of the cycles. To accomplish this, follow the below example (which uses a noninvasive blood pressure graph):
Excluding Noise or Artifact from Analyses that use Find Cycle Operations
- Locate an area of bad or missing data.
- Select an area just before or after the bad section, making certain that enough data has been selected to include at least one full cardiac cycle.
- Use “Min” and “Max” measurements to determine the smallest and largest values.
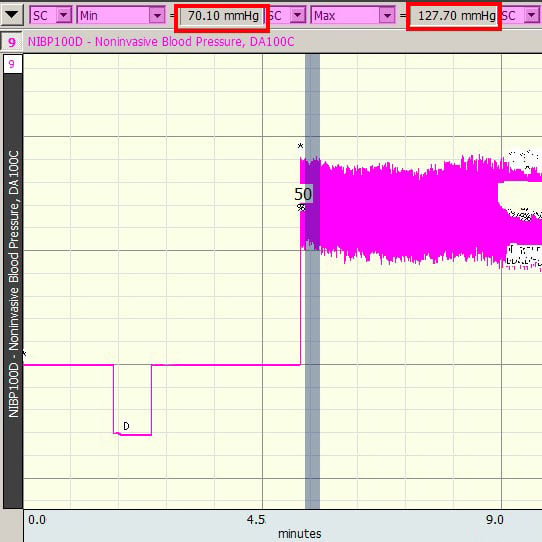
- Select the bad section, making sure that the selected area fully encompasses any values that were lower than the previously determined Min and/or larger than the previously determined Max.
- Choose Transform > Expression… and enter a value midway between the “Min” and “Max” values from the good section of data.
- For this example, 98 was used:
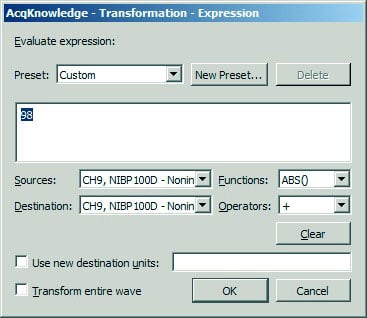
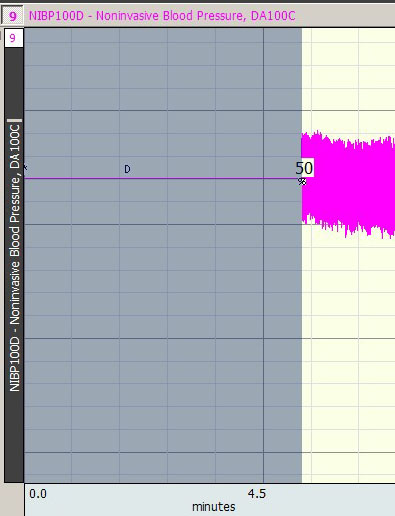
- For this example, 98 was used:
- Repeat previous steps for any other bad areas.
After processing all “bad” areas, analyses that use Find Cycle (such as ABP Classifier) should yield the proper results.
Stay Connected