Airflow Accessories for the TSD117A-MRI:
- For air flow and lung volume measurements, use the TSD117A-MRI with the AFT36 bacterial filter with integrated mouthpiece.
- For measurements of expired gases, use the TSD117A-MRI with the AFT22 non-rebreathing T valve with AFT10 facemask and the AFT15A or AFT15B mixing chambers or the AFT35-MRI low-clearance airflow interface.
Recommended sterilization: cold sterilization (i.e., Cidex) or autoclave. The detachable flow head (RX117A-MRI) is dishwasher safe.
See SS11B for the Telemetry System equivalent.
Sample Setup for Human Airflow in MRI
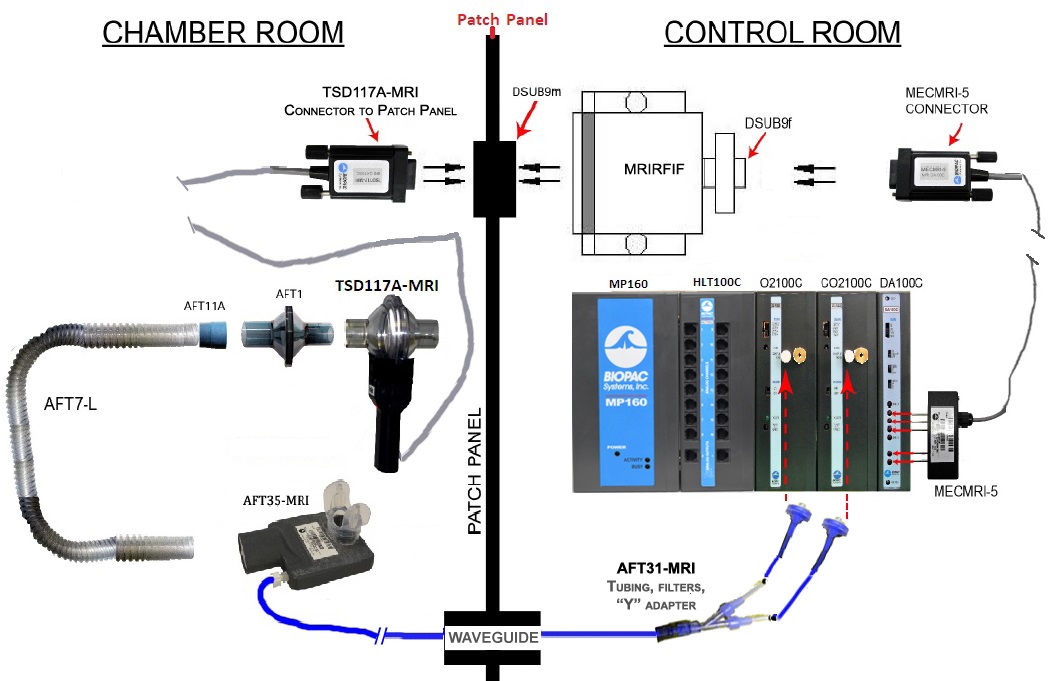
Part #: TSD117A-MRI




Stay Connected