Low clearance—only 25 mm between subject and coil
Dimensions: 25 mm breathing port height (excluding mouthpiece) x 35 mm outlet port diameter x 83 mm wide x 115 mm long
*Head coil fit: Fit is fine with 32-channel head coils; to encourage a better fit in 20-channel and 64-channel head coils, a shortening (cut via snips) of the flexible snorkel mouthpiece may be required.
Deadspace: 88 ml
Materials: Thermoplastic body; silicone inlet filter and mouthpiece; nylon male Luer; polypropylene female Luer
Sterilization: Cidex® recommended
Sample Setups
Perform a variety of tests. Place transducer outside the bore in the MRI Chamber Room and run tubing to connect to the subject and breathing accessories; place amp in Control Room.
- End Tidal CO2: C02100C amp + AFT31-MRI tubing + AFT35-MRI airflow interface
- Airflow & Lung Volume: DA100C amp + MECMRI-DA cable/filter set + TSD117A-MRI transducer + AFT11A coupler + AFT7-L tubing + AFT35-MRI
- Airflow & Lung Volume with End Tidal CO2: DA100C + MECMRI-DA + TSD117A-MRI + AFT11A + AFT7-L + AFT35-MRI + AFT31-MRI + CO2100C
- Metabolic: DA100C + MECMRI-DA + TSD117A-MRI + 2 x AFT11A + 2 x AFT7-L + AFT35-MRI + AFT31-MRI + AFT15A/B + C02100C and/or O2100C
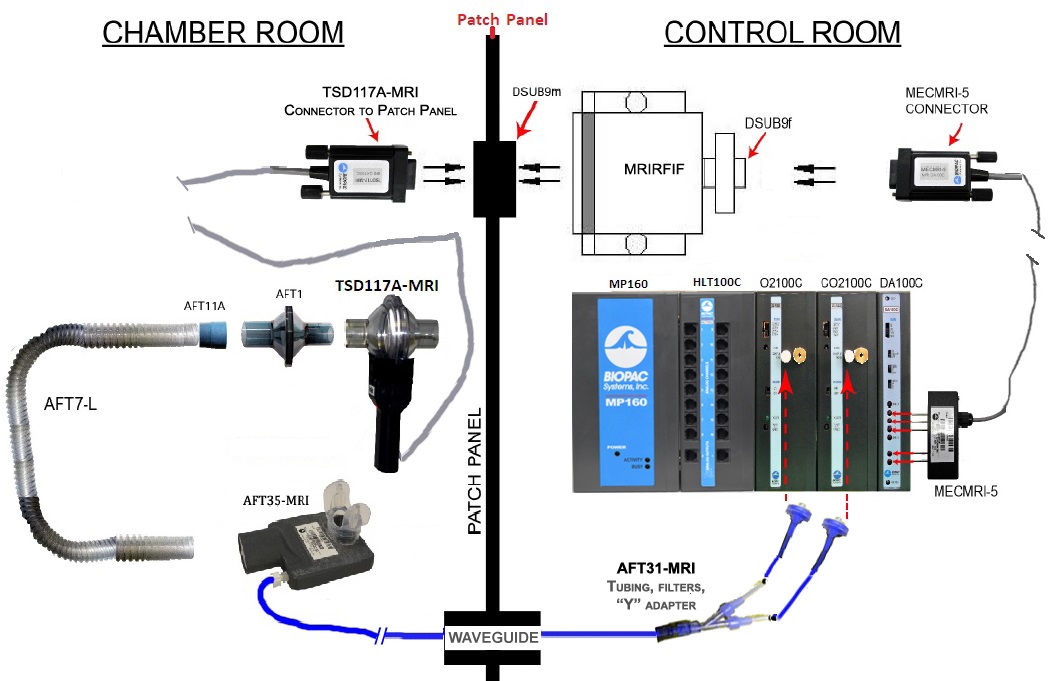
Part #: AFT35-MRI










Stay Connected