Components
The VVK100-SYS includes
- MP160WS/W 16-channel Data Acquisition & Analysis system for Windows or Macintosh. The MP160 system can monitor eight ventilators (pressure, volume), simultaneously.
- TSD157B-MRI-01 laminar flow transducer with Amplifier (DA100C) for precision ventilator bidirectional airflow measurements; TSD157B-MRI-01 consists of TSD160A pressure transducer + RX157 laminar flow head (±120 LPM) with silicone tubing (1 m). Can be inserted, in-line, to typical hospital ventilator systems. Airflow transducer calibration and verification is straightforward.
- AFT27 3-liter Calibration Syringe is included for calibration and validation and is certified to meet or exceed an accuracy of 0.5% (3 liters ±0.5%).
- TSD160D Differential Pressure Transducer with Amplifier (DA100C) that can interface with any pneumatic circuit to monitor airway pressure. When combined with the TSD157B-MRI-01, it is possible to monitor airflow and airway pressure to provide the user with real-time validation data of pump volume and pump pressure ranges.
- AcqKnowledge software controls the hardware, displays the data, and analyzes the signals in real time.
*Optional Add-ons for Additional Measurements
- Real-time Data Access: Data is available in real time for further third-party analysis by using the optional Network Data Transfer licensed feature, providing your systems with immediate network access to the data while the validation process is taking place.
- Oxygen concentration: % Oxygen measurements—synchronized with flow cycling, use TSD301 Galvanic Oxygen Transducer + DA100C Transducer Amp; inline coupler AFT301 available—fits O2 sensor and 22 mm tubing.
- STPD results: STPD results are needed to satisfy ISO Standards (e.g., ISO 80601-2-12). STPD reporting is the only method that permits ventilator performance comparison no matter altitude, temperature or humidity. To convert volumes from ATP (Ambient Temperature and Pressure Dry) to STPD (Standard Temperature Pressure Dry), use:
1) TSD302 Wide Air Range Temperature Transducer (in sampled flow stream)
2) TSD303 Barometric Pressure Transducer (in ambient air)
3) TSD304 Humidity Transducer (in sampled flow stream)
4) TSD305 100 PSI Gauge Pressure Transducer
- Water vapor pressure: use TSD302 temperature sensor, TSD303 barometric pressure sensor, and TSD304 relative humidity sensor.
- Differential Pressure Calibration: Use the AFTCAL-160 NIST certified Differential Pressure Manometer (Range ±2 psi, ±140.6 cm H2O) to calibrate the TSD160 series of differential pressure transducers.
- CPAP mode validation: use the TSD157B-MRI-01 laminar flow transducer included with the system for very low flow and unidirectional measurements (less than 50 L/min).
- Flow testing over multiple dynamic ranges: Contact BIOPAC to review specific testing requirements and recommended physical configurations.
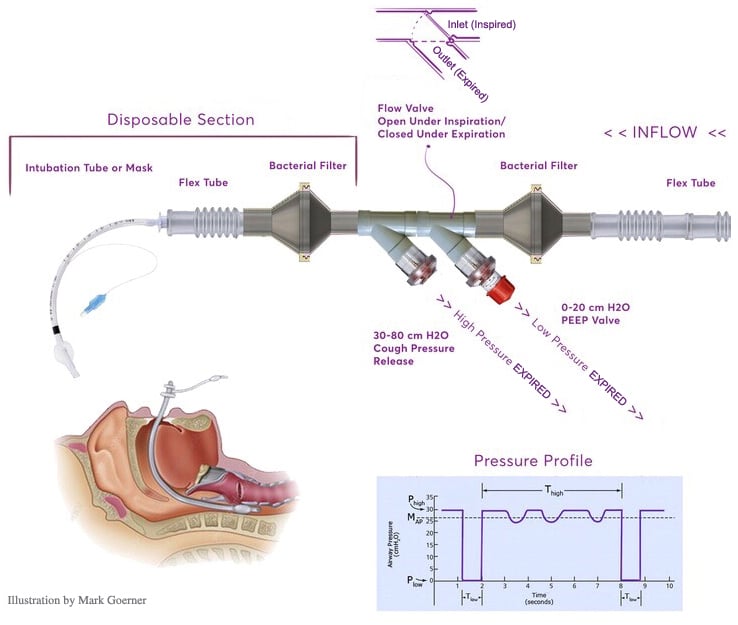
- Interfacing Accessories: BIOPAC offers a wide range of tubing, adapters, bacterial filters, valves, and related accessories. For example, AFT17 tubing will interface between the TSD160D transducer and the ventilator breathing circuit.
Setup

Snap the BIOPAC modules together: MP160 – AMI100D – DA100C (for TSD157B-MRI-01) – DA1000C (for TSD160D) – optional DA100C (for TSD301)
Connect the TSD157B-MRI-01 Laminar Flow Transducer to a DA100C Amplifier.
Connect the TSD160D Differential Pressure Transducer to a DA100C Amplifier.
optional Connect the TSD301 Galvanic Oxygen Transducer to a DA100C Amplifier.
Connect the Transducers to the ventilator circuit (add tubing, filters, etc. as required).
Technical Application Reference
- The Ventilator Validation & Testing Technical Application Reference covers how to connect the ventilator validation kit (VVK100-SYS) to a standard, typical ventilator to allow for its verification, ventilator test calibration, and measurements for ventilator validation: Pressure and Timing; Cough Pressure Release (obstruction valve); Positive End-Expiratory Pressure (PEEP); Pressure; Oxygen Concentration; Air Flow & Volume.
BIOPAC products are used by 99% of the world’s top universities
and have been cited ~51,500 times.
Product Family
VENTILATOR SUPPORT
- Ventilator Field Verification
- Failure Analysis
VENTILATOR PRODUCTION
- Ventilator Manufacturing Testing/Validation
- NDT for Automated Test Analysis
- Troubleshooting
VENTILATOR DEVELOPMENT
- Ventilator Pressure and Flow Measurements
- Relief and PEEP Valve Validation
- Flow and Pressure Regulator Evaluation
- Cycle Timing Analysis
- Leak Detection
- Ventilator Mode Analysis (ACV, SIMV, PCV, PSV, APRV, etc.)
- Oxygen Percentage Measurement*
- Temperature and Humidity Measurement*

















Stay Connected