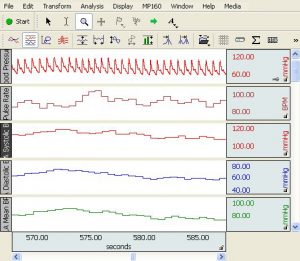
 Parameter classification: Sys, Dia, Mean [mmHg] | Pulse [bpm]
Parameter classification: Sys, Dia, Mean [mmHg] | Pulse [bpm]
Inflation pressure: Typical 120 mmHg (16 kPa) | Min. 30 mmHg (4 kPa) | Max. 300 ±10 mmHg (41.3 kPa ±1.3 kPa)
Measuring range: Systolic 40 – 250 mmHg (5.3 – 33.3 kPa) | Diastolic 30 – 210 mmHg (4 – 28 kPa) | Mean 35 – 230 mmHg (4 – 30.6 kPa)
Heart rate indication range: 30-200 bpm
Excess pressure limit: 300 ±10 mmHg (40 kPa ±1.3 kPa)| Response time: < 3 sec. | Deflation time: < 15 sec | Protection against electric shock:
Type BF Display resolution: 1 mmHg (0.1 kPa)
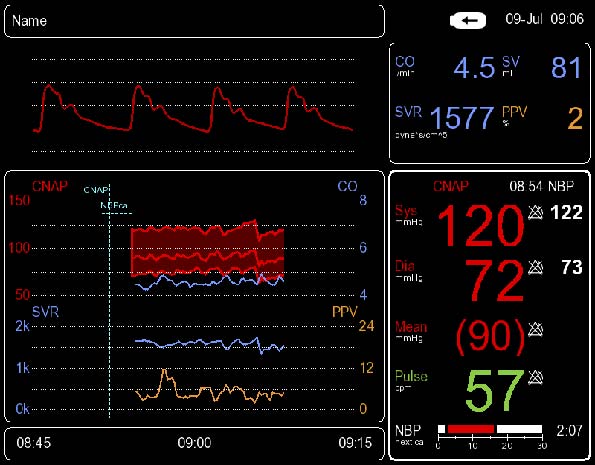
Measuring ranges
CO: 0,0 – 99,9 l/min
SV: 0 – 500 ml
SVR: 0 – 9999 dyne*s/cm5
PPV: 0-40%
CI: 0,0 – 99,9 l/min/m2
SVI: 0 – 500 ml/m2
SVRI: 0 – 9999 dyne*s/cm5/m2
SVV: 0-40%
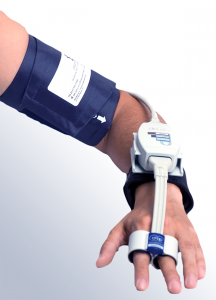 Set-up: Simple and Quick
Set-up: Simple and Quick
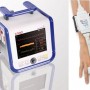
- One finger sensor provides all parameters noninvasively–no placing of catheter or additional electrodes
- Comfortable for subjects in short or long‐term studies
The NIBP100D-HD system is very user friendly and the initial setup and calibration period takes less than three minutes—that time includes placing the cuff around the upper arm and the sensor on the fingers. Placing the finger sensor is as simple as sliding the subject’s fingers through the two cuffs. See the Set Up Guide under the Support tab for more information.
Recording: Immediate Feedback
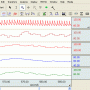
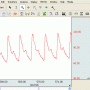
- Real‐time, continuous, noninvasive blood pressure displayed only shortly after startup
- Enables accurate & immediate feedback on BP, PR
- Proven solution for consistent, repeatable results
Interface: Easy Data Transfer and Analysis with NIBP100D-HD
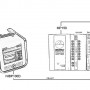
Interface with BIOPAC’s MP160, MP150, or MP36R data acquisition systems or a third‐party data acquisition system.
Interface cable CBLNIBP100D-HD is included with every NIBP100D-HD System; additional cables required based on system that is interfaced and if electrical isolation is required.
- One end of the CBLNIBP100D-HD cable connects to the “AUX” port on the right side of the NIBP100D-HD unit
- The other end terminates in 4 x 3.5 mm male connectors, labeled CH 1 BP, CH 2 MAP, CH 3 CO, and CH 4 Pulse, compatible with firmware version 5.2. (Cables shipped prior to June 2016 are labeled BP, MAP, CO, and PPV, and are compatible with NIBP firmware 5.0).
- BIOPAC recommends 4 x INISOA for optically isolated connection (electrically safe inputs). Optionally, 4 x CBL122 can be used when it is not necessary to provide electrically safe inputs (for example, when no participant is connected to the equipment at the same time via wired leads).
MP160 System: Connect CBLNIBP100D-HD to the AMI100D included with current MP160 Systems or to the HLT100C “Rev 2” shipped with older MP160 Systems. Add 4 x INISOA for isolated (electrically safe) inputs.
Important! HLT100C Rev # is indicated on the part#/barcode label: “Rev 2″ units shipped with older MP160 Systems and cannot physically be used with an MP150+UIM; older “Rev 1” units shipped with MP150 Systems.
MP150 System: Connect CBLNIBP100D-HD to HLT100C (Rev 1); Add 4 x INISO for isolated (electrically safe) inputs.
MP36R System: Add 4 x BSL-TCI5 mod phone jack interface to connect CBLNIBP100D-HD to the CH ports on the front of the unit.
Safety Note When connecting this system to an A/D system, be aware that the outputs are NOT electrically isolated and will need to be isolated before going into the A/D system. This will keep the subject electrically safe. This is especially important when connecting electrically via ECG, EMG, EDA, EEG, etc. to the subject.
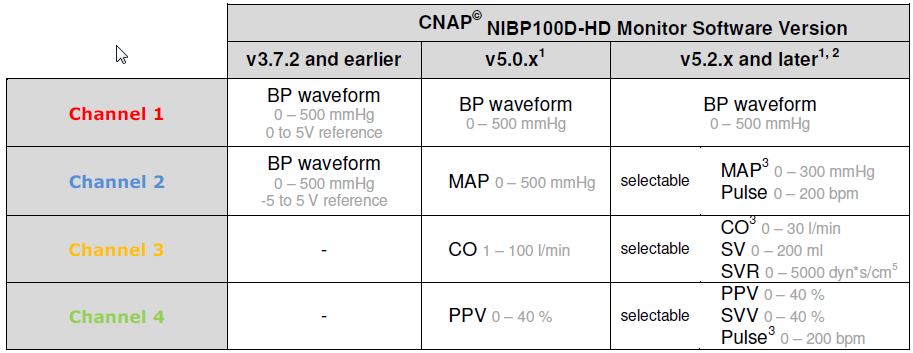
1: Starting from version 5.0.x the output voltage range (reference voltage) can be selected by the user between 0 to 5 V (default) and -5 to 5V (see operator’s manual – chapter 4.6.1); 2: Since version 5.2.x the parameter output on channel 2, 3 and 4 can be configured by the user in the menu Setup | Measurement | Output Options; 3: Default setting
See Also
- Continuous non-invasive arterial pressure shows high accuracy in comparison to invasive intra-arterial blood pressure measurement
 (Sackl-Pietsch E., Department of Anesthesiology, Landeskrankenhaus Bruck an der Mur, Austria)
(Sackl-Pietsch E., Department of Anesthesiology, Landeskrankenhaus Bruck an der Mur, Austria) - Continuous non-invasive blood pressure monitoring using concentrically interlocking control loops. J. Fortin, W. Marte, R. Grüllenberger, A. Hacker, W. Habenbacher, A. Heller, CH. Wagner, P. Wach, F. Skrabal Computers in Biology and Medicine – September 2006 (Vol. 36, Issue 9, Pages 941-957, DOI: 10.1016/j.compbiomed.2005.04.003)
- BIOPAC blog: Ensuring Error-Free NIBP Measurements and Data
- BIOPAC blog: Noninvasive Hemodynamic Monitoring in Research







Stay Connected