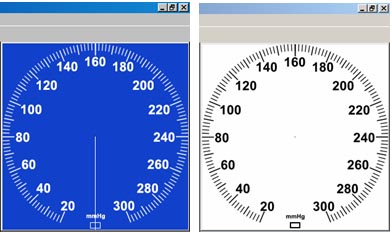
Gauge color can be set under Lesson Preferences.
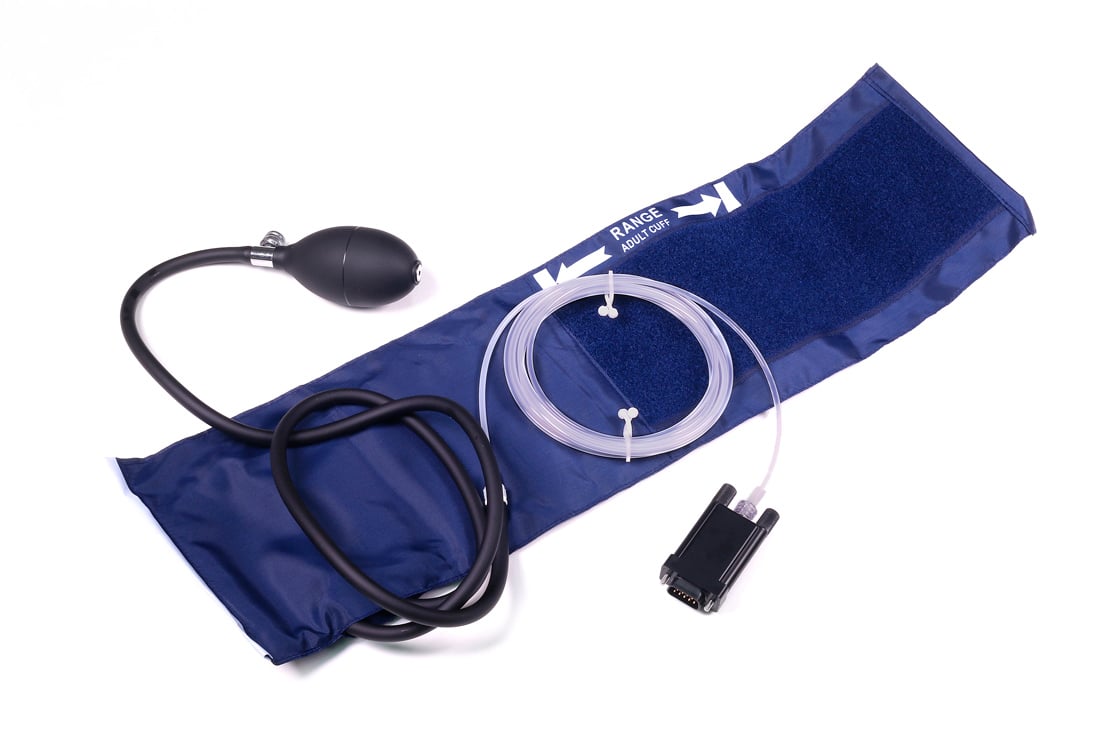
Use the BP Cuff transducer with the SS30L stethoscope for the evaluation of systolic and diastolic blood pressure. When the stethoscope is used to record the Korotkoff sounds it is easy to obtain very accurate and repeatable results. For alternate cuff sizes, see the RX120 Cuff Series.
Note – SS19LB/LA is not compatible with MP45 Systems (USB chip conflict); use SS19L with MP45 Systems.
Calibration
The built-in pressure transducer requires an initial calibration. see specs for detailed instructions.
Part #: SS19LB, SS19L




Stay Connected