EDA FAQ
EDA Questions and Answers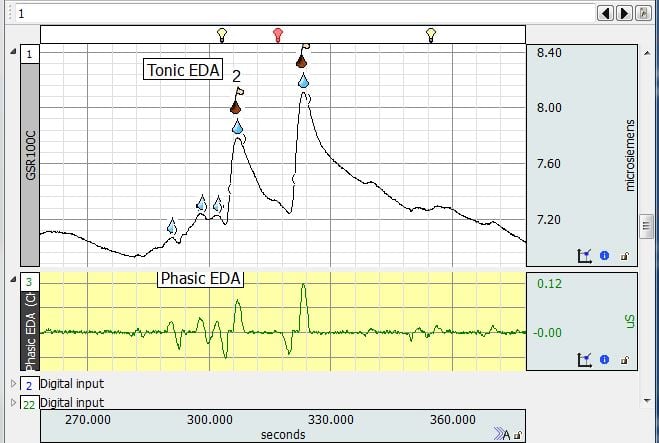
Your colleagues submitted these questions during BIOPAC EDA Webinars.
Topics include
- EDA Skin Prep/Gel Application
- EDA Electrode Placement & Grounding
- EDA Recording Systems
- EDA Data Measurement & Analysis
- EDA Artifact Detection & Correction
- Temporal Factors and EDA
- Stimulation and EDA
- Recording EDA in the MRI
- EDA Markers
- EDA Recording: Non-responders
- Other
If your question isn’t covered, learn more in EDA: Electrodermal Activity Applications, review EDA Signals & Measurements, or contact BIOPAC Support.
EDA Skin Prep/Gel Application
- Q: What is the process to put on the electrodes on the participants to get a good signal? Should I have them wash their hands? Use alcohol? Conductance gel?
A: No alcohol should be used as it dries out the skin. If the participant must wash their hands, use just plain water. The disposable electrodes (EL507 and EL509 for MRI) are already pre-gelled, but if they are dry, GEL101 isotonic gel must be added. When using reusable electrodes, such as the TSD203, fill the cavity with GEL101.
- Q: Isn’t it important to control the area of contact? If gel covers a larger surface area, conductance levels will be higher.
A: With both disposable and reusable electrodes, the surface area remains quite consistent between participants. For example, the glue of the EL507 and EL509 electrodes is quite strong so the gel cannot really spread beyond the contact area of the electrode once you apply it to the skin and the sponge pad helps to keep the gel in place.
- Q: Do we need to apply the gel also on the electrodes for one use? How much? Do we apply it on skin or on electrodes? Thanks.
A: If the electrodes are fresh, you do not have to do anything. If they have dried out (you will know it, as they will feel dry to the touch and may even have a crust) then add gel as per the procedure that was described during the webinar.
- Q: Is there any downside (other than using up GEL101) to “reviving” electrodes that have not yet dried out? In other words, erring on the side of caution.
A: There is a downside. Since you are mixing the fresh gel with the dried gel, you are altering the salinity. Thus, the resulting gel mix will be more likely to saturate the sweat glands. Placing extra gel on the electrodes is a temporary solution until you can obtain new fresh electrodes.
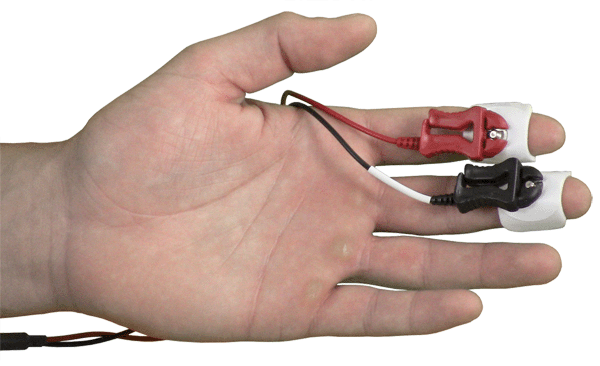
Disposable EDA Setup: SS57LA leads + EL507 EDA electrodes
- Q: How does pressure applied in the electrodes area affect EDA?
A: If you are using the TSD203 EDA transducer or SS3LA reusable electrodes, you want to avoid over tightening the strap because you may occlude the vessels in the finger. The Velcro strap should be just tight enough to hold the electrode in place and prevent it from moving during the recording session. If you are using disposable electrodes EL507, you can also use surgical tape to ensure that the electrodes remain attached to the participant but the tape should not be applied too tightly, just tight enough to hold the electrode in place.
- Q: When a participant’s hand is cold, there is no response or the EDA data looks very strange. What are the recommendations on subject and room temperature settings during data collection with EDA?
A: Subjects with cold hands will not normally provide good EDA data. Room temperature should be ambient between 22-240C—not so cold as to have the subjects feel chilled, not so hot as to have the subjects sweat. Either of these conditions will adversely impact the quality of data.
- Q: How about some participants who have cold hands? Does their temperature of hands influence recording EDA data?
A: Yes, we have observed that cold hands will negatively impact the quality of EDA data, reducing the SCR size in some participants. This subject is also discussed in detail in the Handbook of Psychophysiology.
- Q: Is it just the reusable electrodes that dry out, or do the SS3L electrodes dry out as well?
A: The SS3L EDA transducer contacts are filled with gel at the start of the study, then they are cleaned and stored away. Disposable electrodes are pre-gelled and if they are not stored properly or they are past the expiration date, the gel may be dry.
EDA Electrode Placement
- Q: Electrode placement table—what’s the paper title?
A: Emotional sweating across the body: Comparing 16 different skin conductance measurement locations, Physiology & Behavior 106 (2012) 298–304
- Q: What are the ideal locations for the FPS (startle) electrodes?
A: It is best to refer to the Committee report on Guidelines for human startle eyeblink electromyographic studies.
- Q: What about electrode placement on thenar and hypothenar?
A: The Committee report on Publication recommendations for electrodermal measurements, just like the Handbook of Physiology, refers to this and the volar phalanges placement as recommended. It does not compare the two, however, so I recommend reviewing the literature to see if there is an indication which placement is the best, thenar and hypothenar or volar phalanges. It is hard for us to make a recommendation when there are so many variables.
- Q: Is it possible to use ECG electrodes at the palm of the hand?
A: ECG electrodes should not be used to record EDA because the gel is not isotonic.
- Q: Electrode placement. I have used some of the systems that purport to record from wrist. Doesn’t seem to work.
A: Please see the presentation for recommended and documented areas for electrode placement.
- Q: I’m particularly interested in getting better data from elderly participants.
A: The main issue will be ensuring that you have good electrode contact with the skin. Make sure that you are using fresh electrodes that have plenty of gel and test the subject by asking them to take a breath and hold it. The electrodes should be on the participant for at least 5 minutes and this may take a little longer with some subjects. Poor peripheral circulation will also have an impact on the quality of the signal. The import thing is to test the subjects before starting the recording and making sure that you are getting good responses from them. I would also consult the literature to see if there are specific recommendations for your study population.

Reusable EDA Transducer: TSD203 or SS57L
- Q: What about recording EDA from the underside of toes with the TSD203 electrodes?
A: The instep of the foot and the palmar surface of the hand have the highest concentration of sweat glands on the body and are thus ideal locations to record from.
- Q: When I check the electrode setup with EL-CHECK, I always get the red or orange light as a result—is this normal? If not, what can I do to get a better signal?
A: Impedance checking is not necessary for EDA. Isotonic GEL101 for EDA recording is not as conductive as regular electrode gel, such as GEL100, and values will naturally be higher. If you are asking how to improve the impedance for biopotential recordings, such as EMG, EEG, etc. then the following will help: abrade the skin using ELPAD or place the electrodes 5-10 minutes in advance. For a full list of recommendations, see Tips for Recording Good Data.
EDA Grounding
- Q: When using other measurements with EDA, for instance ECG or EMG, which one should be used with the lead? Does it matter, or is it better to have it on a particular measurement?
A: The subject is grounded by the EDA amplifier. This means that you do not have to add an additional ground on the other biopotential signals – ECG or EMG. However, if you would like to run an additional ground, without creating a possible ground loop, you should use one of the CBL205 adapters. The CBL205 plugs into the ground on the biopotential amplifier and then the ground electrode lead plugs into the other end of the adapter.
- Q: I have a question about safe grounding. We often collect ECG and ground the subject with three lead set up that way. We don’t ground on any of our other channels. But your slide suggested that we need to have a separate filter to place in between the lead and the EDA amplifier for safety. Please elaborate in the Q/A document.
A: When using EDA, you are already grounded via the VIN- connection of the EDA100C/GSR100C amplifier. Thus, no other ground is necessary, not even an ECG ground. However, you can certainly use other grounds, just make sure to use a CBL205 cable at the ground lead connection for any additional grounds you may want to use.
A second ground can be very useful when recording EEG or EMG (because the quality of the ground connection at the finger with isotonic gel is not the best possible and for these sensitive signals it’s nice to have a separate ground) or in the event that the EDA lead gets disconnected through movement artifact, etc.
- Q: What do you recommend if the participant is sweating (and some people sweat more than others) over the course of activity for which we are collecting EDA for?
A: The hands are not a principal site for thermoregulatory sweating so the impact on recorded EDA should be minimal.
- Q: What are the best electrodes to use with DC voltage? Which electrodes do you use with EDA100C? How long AgCl electrodes (if needed) could be used with the DC system? Can you use 3M Red Dot Monitoring electrodes? How should we apply gel on the electrodes?
A: Please review the section on gel application to electrodes. We strongly recommend TSD203 (MP150) or SS3LA (MP36R) reusable electrodes or EL507 disposable electrodes (use only EL509 electrodes for recording in the MRI). These have been extensively documented in multiple papers. We also strongly recommend GEL101, as other gels are not isotonic and will have a salt concentration which may impact data quality. Dry electrodes may be salvaged—see the presentation—but for best results we recommend using fresh electrodes.
- Q: So should a ground electrode be used (in addition to the VIN-) if the EDA100C is the only amplifier being used?
A: No, an additional ground electrode is not required when recording EDA. The subject is automatically grounded through the EDA electrodes. If you want to use an additional ground electrode, use the CBL205 in series with the ground cable of the other amplifier. This is not typically recommended when only recording EDA. Please read this Grounding Notes.
EDA Recording Systems
- Q: Is there an expected difference in reliability of the data between recording EDA in the lab with a MP150 and with a mobile device. We have compared the devices at the same time point and the data doesn’t really look the same.
A: Data recorded from the same electrodes will look identical with one exception: the wireless system uses 12-bit A/D conversion before broadcasting the data, while the wired system uses 16-bit A/D conversion. This means you can detect much smaller changes in skin conductance with the wired system. The minimum resolution with the wireless system is 0.012 µS, while with the EDA100C amplifier it is several orders of magnitude smaller (because you can also change the gain and zero offset of the amplifier). For practical purposes, the resolution of the wireless system will be enough, but this is something to keep in mind if extremely sensitive measurements are necessary.
- Q: What specs (pertaining to validity & reliability) should researchers be aware of when comparing wearable mobile tech devices?
A: One of the best references is to look for publications that have employed the device and technology. BIOPAC products have been cited more than 51,500 times and over 2,630 times specifically for EDA. I would also check how the device is measuring the signal of interest and make sure that the technique is providing you with the data you require for the application. Many wearable devices are great for consumer use but not really suitable for research purposes. The BIOPAC BioNomadix product line has been proven in the lab and is now available for remote subject monitoring.
- Q: Any differences between M35 (previous system with non-disposable EDA electrodes) & my new system (M36) with disposable EDA electrodes?
A: The following link will provide you with information about the MP36R hardware.
- Q: Hi, I was just wondering how the hardware setup changes if you are using the TEL100C telemetry system?
A: Just like with the EDA100C/GSR100C amplifiers, make sure you are in DC mode. You should also make sure the gain you are using is appropriate. Please contact us at support@biopac.com if you have specific questions.
- Q: Can you record several videos simultaneously with AcqKnowledge?
A: Using the CAMSYS4 and CAMSYS8, you can record and synchronize 4 or 8 videos respectively. Furthermore, using the OUT103 led as a sync marker, there is no limit for how many external video cameras you can synchronize with AcqKnowledge and later link the footage to the software. See here. In addition to the multi camera setups there is also a High Frame Rate option for recording fast events.
- Q: Will you be including MP36 systems?
A: The analysis approaches apply equally to data recorded with the MP36 system. The MP36R system comes with the AcqKnowledge software, which was used for the presentation. The BSL software comes with the regular MP36 system (part of our educational BSL package) but has fewer features as it is designed for teaching purposes. AcqKnowledge can open files created with the BSL/MP36 system and, therefore, you can analyze BSL data using the more advanced research software, AcqKnowledge.
- Q: Is it possible to use the BioNomadix system with the MP36 system?
A: It is possible, but please contact us so we can discuss your options.
- Q: Can an EMG headset be used to record wireless EDA data?

BioNomadix Wireless EDA
A: No. EMG equipment records a biopotential voltage while EDA works by passing a current through the body and recording conductance. We have the BN-PPGED module to accomplish wireless EDA recordings.
- Q: Does the BioNomadix wireless logger with PPGED-T transmitter require calibration?
A: The devices are factory calibrated but you can confirm the calibration by using known resistor values. Consult the Support Department for further information.
EDA Artifact Detection & Correction
- Q: Can you elaborate on how to identify electrical noise in the recording? As well as how to fix it when the participant is there or during the data cleaning stage?
A: Electrical noise is 50 Hz or 60 Hz. You can use the Spectrum analyzer palette (Display >Show >Spectrum analyzer palette) in real-time as well as after the recording. A peak will be obvious at 50 Hz/60 Hz (depending on your region). This is an unusual problem to have with the EDA data as it is low-pass filtered at the amplifier. This means the source of noise is not related to the EDA, with the most likely culprit being third party equipment that is connected to our system without optical isolation. Dry electrodes or poor contact between the electrode and the subject will also exasperate this problem.
- Q: How do I remove TTL artifact from GSR data prior to copying graph data?A: If you have the TTL triggers going to the digital channels on the system, you should not have artifacts on the raw data. Are you sure the artifacts are coming from the TTL triggers? If you review the earlier EDA webinar, we cover the use of the AcqKnowledge’ s, median smoothing function. This tool should allow you to remove such artifact without impacting the raw data. The median smoothing tool is a wonderful way to eliminate random spikes from slow moving data such as EDA. The following link will take you to the webinar: https://www.biopac.com/events/eda-webinar-part-1-recording-great-data/
- Q: You said fast SCL rise equals good data, is “fast drop of SCL” can be seen as a bad data (artifact)?
A: What’s important is to be aware that a SCR reaches the peak over 1-3 seconds while 50% decay may take anywhere from 2-10 seconds. So skin conductance data rises much faster than it drops. For instance, a very fast drop, for example a full 1 µS over less than 2 seconds, is unlikely to be physiological in nature.
- Q: For artifact correction: Should median smoothing be done before low-pass-filter or the other way around?
A: The median smoothing should be performed first to remove any high frequency artifacts from the signal before low pass filtering it. Low pass filtering will smooth artifacts into the data, so they should be removed first.
- Q: Where can I find some instructions on how to automate (i.e., generate a script) artifact correction?
A: Scripting is covered in this EDA Analysis & Scripting webinar.
- Q: Do you have any suggestions for removing artifact from mobile subjects?
A: It’s best to avoid artifact in the first place by using fresh electrodes and taping over the leads and electrodes. Experiment with placing electrodes in alternate locations that will not be influenced by movement that much but explore the literature beforehand. We have included one such reference in the presentation. If you are already done with the experiment, please refer to the answer of question 11. We are also working on new strategies for mobile applications that will help to prevent or minimize artifacts created during mobile recordings.
- Q: Please explain the automated artifact removal scripting in detail or provide additional information for this feature! Thanks!
A: This is discussed in detail during the follow-up webinar.
- Q: How do I recognize and remove artifacts created by movement?
A: These are changes in skin conductance that do not make sense given physiological expectations. Typically this means the EDA goes up or down at a very fast rate. Such artifacts are very fast. Here is an example:
Next, we have resampled the data to 50 Hz and applied a 50-sample median smoothing filter (Transform->Smoothing) and then 1 Hz FIR low pass filter (Transform > Digital filters > FIR filter > Low pass). We have zoomed in and the original data is seen on top with the transformed result on the bottom. The artifacts are completely eliminated:
This is even better illustrated by showing the waveform in scope mode, with the green waveform representing the clean result:
This is an example of the most typical type of artifact and method of correction. However, we will release an EDA troubleshooting guide in the future that will include AcqKnowledge sample files of various types of issues so you can learn how to handle the data yourself.
The follow-up webinar will address artifact removal automation techniques.
- Q: How do we quickly diagnose and fix low EDA signals: What to do when reading starts nice and smooth and then goes fuzzy? What causes the interference or artifacts that look like rectangular drops in the signal, and what is the best way to avoid/remove them? I noticed this in the data sample “untitled 3” that you had open at the very beginning. There is a lot of noise in my signal (e.g., the line is “hairy”). Is this something that will impact my data analysis?
A: The most common causes of low signal or poor data are poor electrode placement (location and/or adhesion) or the subject banging or knocking the electrodes. The subject banging or knocking the electrodes can also cause artifacts that are clearly not EDA. The presentation, along with BIOPAC Application Notes and Knowledge Base provides references on how to prevent issues from electrode placement. Use of a low-pass filter set to 1 Hz, as noted in the presentation, can help address some of the issues with “fuzzy” or noisy data. Artifact identification and removal (including motion artifacts), will be covered in the EDA Analysis Essentials Webinar and is covered in the recorded EDA Analysis and Scripting Webinar.
- Q: Can one use ICA to remove artifacts?
A: There are many techniques that can be employed to remove artifacts but we try and avoid spending too much time removing them by placing the electrodes and leads in locations that are less problematic. The tools in the webinar will provide you with some good examples for rapid data cleanup.
- Q: Respiration artifact correction! How to decipher a real SCR from a resp-related SCR?
A: When we test the EDA signal, we ask the participant to take a sharp, deep breath and to hold it for a second. This process will result in a response from most subjects. However, it is unlikely that a subject will perform this maneuver under normal circumstances. Normal breathing patterns will not result in a response but you can always record the respiration signal and check to see whether participants have irregular breathing patterns that may be influencing the data.
- Q: Is there a way to salvage data that is messy because of substantial movement/messing with the electrodes? For example, in our study on SCR among anxious participants our most anxious participants were the ones who played with the electrodes the most making their data (which is of most interest) also the messiest.
A: There is a lot that can be done to recover the data. You can use the median smoothing, connect endpoints and filtering techniques and/or contact us at support@biopac.com so we can help with further suggestions. We have seen literally thousands of files with EDA and can usually help to extract some useable data. However, it makes sense for you to consider using alternative electrode locations where participants are less likely to play with the electrodes and leads.
EDA Data Measurement & Analysis
- Q: How do I preprocess and analyze my data sets?A: I would recommend watching some of our earlier webinars because they cover recording and preparing the data for analysis. The following link will take you to the webinar section of the website. https://www.biopac.com/webinars/
- Q: What is EDA signal? How it is being recorded? Why do we need to record EDA instead of recording EMG? What is the relation between SCR, EDA, GSR? What is the difference between EMG & EDA?
A: A good discussion and differentiation of these signals can be found in Handbook of Psychophysiology. John T. Cacioppo, Gary Berntson, Louis G. Tassinary and Psychophysiology: Human Behavior and Physiological Response by John L. Andreassi.
A: The webinar covers everything from subject, hardware and software setup, to recording great data and analyzing the signals.
- Q: How should we automate preparation (e.g., cleaning) of EDA data, checking data quality, and filtering?
A: The presentation provides a good overview of the recommended steps for preparation of data and examples of good vs. bad data. The recorded EDA Analysis and Scripting Webinar shows how these functions can be automated using BIOPAC scripting. These functions will also be addressed in the EDA Analysis Essentials Webinar. Finally, we recommend you consult the EDA resources in our Knowledge Base and Application Notes.
- Q: GSR peaks indicate emotional arousal. They are identified by eyeball inspection. How can such peak identification be automated?
A: The webinar demonstrates two automated techniques for identifying and scoring Skin Conductance Responses. AcqKnowledge includes fully-automated routines for EDA analysis.
- Q: Different ways of analyzing EDA data (tonic vs. phasic responses)
A: The presentation shows how to acquire both the Tonic and Phasic signals and walks through a variety of analysis options.
- Q: Do you have any tips for using AcqKnowledge to compare background tonic SCL across multiple periods within a file?
A: The Focus Areas are a great tool for comparing segments of data. I demonstrated the use of the Focus Areas in the webinar and they were also covered in the previous webinar. We also have a screencast that provides additional information. https://www.biopac.com/events/acqknowledge-webinar/ , https://www.youtube.com/watch?v=CBYGYklB9Yo
- Q: We record continuously remotely we want to segment periods of interest and compare reactivity scoring within and across controlsA: If you want to manually identify the periods of data, you can use the Focus Areas to highlight the regions you want to analyze. If you use the SCR analysis routine, it will score the data and you can then use the Epoch analysis to take measurements from the Focus Areas.
- Q: How to batch mode analysis in AcqKnowledge?
A: The following link will take you to a webinar that covers analysis and scripting. The BIOPAC basic scripting tool allows you to batch process analysis and automate much of the analysis that were included in this webinar: https://www.biopac.com/events/eda-webinar-analysis-scripting/
- Q: What would be the best measure to report for tonic EDA? Frequency, Amplitude or SD of NS-SCR?
A: We refrain from advising on the best measure. We recommend that you consult the reference literature that was included in the presentation as well as the literature on the topic of EDA in general. The analysis routines in AcqKnowledge will provide you with a wide range of options to choose from and all of the frequently used and recommended measures.
- Q: Does the Connect Endpoint function use a linear model to connect the points?
A: It draws a line from the first selected sample point to the last selected sample point and interpolates the values on this line to replace the original data.
- Q: Can I just study using the raw EDA data? Or should I edit the data (e.g., smoothing?) beforehand?
A: If the signal is clean, it can be used as is. But you can resample to 50 Hz (no less than 50 Hz) in order to speed up the performance of the analysis algorithms.
- Q: Why was median smoothing selected? How was the 50 setting selected?
A: Median smoothing rejects outliers while mean smoothing averages them into the result. It will eliminate rap transient spikes from a slow moving signal such as electrodermal activity. 50 samples were selected because the sample rate was 50 Hz. Usually, a smoothing window equal to the number of samples per second will remove most artifacts while not disturbing the physiological trends in the data. Remember to apply a 1 Hz FIR LP afterwards, to smooth out the result. You can perform testing with these and other settings on clean data and compare to the original to see that the data are not significantly altered by the transformations. We recommend such testing steps before transforming data in general. See also Methods for Computing Phasic EDA.
- Q: Will using smoothing on a signal remove some information that might otherwise be useful, such as skin conductance responses or altering the SCL?
A: No, median smoothing will not impact the signal if it is applied correctly and it is easy to determine whether you have altered the signal by overlapping the raw and smoothed waveforms. If the filter becomes too aggressive (if you use too many samples for the window), then it will also transform the underlying trends in the signal. For data sampled at N samples per second, a median smoothing filter with window size N will result in a slight reduction of observed P-P changes in responses:
You have to define the limits for an acceptable transformation. In this example, the peak-to-peak measurement after smoothing and low-pass filtering at 1 Hz differs by about 3% from the raw data (0.337 vs 0.347). But a filter this aggressive will eliminate most fast motion artifacts. If this trade-off is acceptable it’s the researcher’s decision and we recommend to always perform testing on both clean and noisy data before choosing a strategy to remove artifacts.
- Q: You selected “Smoothing Baseline Removal.” If I want to compare the data of baseline (before sound stimuli) and data of sound exposure, should I still choose that?
A: Smoothing baseline removal was used during the webinar as a method to obtain the phasic EDA signal, the signal that represents changes in EDA. If you want to compare the participant’s responses during two different blocks of the experiment (such as when sound stimuli are presented vs baseline), please refer to the section of the webinar that covered the block analysis.
- Q: Can you look at batches of waveforms from different subjects in addition to batch analysis?
A: Yes.
- Q: Are there any sources you can provide for how to run statistical tests using the data obtained from BIOPAC?
A: We would like to refrain from making recommendations on how to run statistical tests.
- Q: Can the analysis (as shown at this moment) be applied to multiple participants without having to do it manually for each participant?
A: Yes, and this will be covered in the follow-up webinar.
- Q: Why resample? Is there a problem with having it at the 2,000 Hz rate of data collection?
A: Reducing the sample rate lessens the computational load for the analysis.
- Q: Is it possible to get access to graphs/pictures of what the EDA signal will look like when different issues arise (e.g., how does EDA look when electrodes have expired, when the wrong gel is used, when the participants are moving a lot, when there is momentary decoupling, etc.).
A: A separate troubleshooting guide is being prepared and it will contain actual data, not just pictures. We should focus on good data—show what the signal should look like and not worry about what bad data looks like. I do not like this approach…there are just too many potential issues.
- Q: We find that while we are able to get a good EDA signal initially, the signal turns into noise within 20 minutes of the protocol. Can you offer any insights?
A: It is best to send over a data sample to support@biopac.com. We would need to see the data to provide useful advice. Please send the raw .acq file. However, I would start by looking at the electrode to subject connection and make sure that everything is good there. Check the quality of the electrodes and ensure they are making good contact with the subject. When you send a file make sure that you include a full description of your equipment and participant setup including any tasks the subject is performing.
- Q: How do you analyze SCR that has both anticipatory and stimulus dependent responses within a short time window?
A: The AcqKnowledge cycle detector can take measurements around specific stimulus events, both before the event and after the event, to automate the extraction of measurements. If you use the automated Event-related EDA Analysis option, the software will identify specific and nonspecific skin conductance responses within the recording. You can then use the Find cycle detector to measure SCL and count SCRs during the anticipatory phase of the recording. We have several Find Cycle screencasts that demonstrate how to use the Find Cycle detector. I would also refer to the guidelines to see what is recommended for measurement timing intervals.
- Q: How would you go about analyzing unspecific EDA data?
A: This will be similar to the Block demonstration I gave in the presentation, but instead of blocks, you can look at responses over defined time intervals – e.g., every 2 minutes. AcqKnowledge has a fully automated Epoch analysis routine that will allow you to automatically measure the data and export the results to Excel. The Epoch Analysis screencast will provide some additional information about this feature.
- Q: I notice a lot of variability among participants’ EDA data, in spite of them all being subjected to the same experimental manipulation. This variability makes it difficult to test the hypotheses even when the sample size is good (e.g., 30-40 participants per group in a 2- or 3-group between-groups design). I am already following the same protocol with all participants; is there anything else I can do to reduce this variability? I observe this outcome when using either MP100 or MP45 system.
A: I’m not clear what you mean by EDA variability because there are two primary signals – Tonic and Phasic. The Skin Conductance Level will vary quite a lot among participants because this is the absolute signal, the tonic waveform. However, the Skin Conductance Response analysis determines how responsive a subject is and also measures the size and amplitude of each response. The software measures the SCR amplitude by measuring SCL at the point of SCR onset, and again at the peak of the SCR, and then subtracting the onset value from the peak value to provide the amplitude value. The size of the response is relative to the SCL at the point the response started. If you are concerned about the quality of your data, you can contact our Support Department and they will gladly provide you with some specific feedback.
- Q: How to standardize and compare one’s EDA data with others?
A: If you are trying to compare data that has been previously published, you can, in most cases, adjust the settings in AcqKnowledge‘s automated analysis routines to match those described in the publication. I would also recommend consulting the list of references that are included in the webinar slide deck to determine current recommendations for EDA analysis. BIOPAC has created an automated solution that conforms to the guidelines but also provides users with flexibility to match other analysis strategies.
- Q: What is the value of viewing the EDA response with a ball pushing a bar rather than viewing it as a graph/in the format it is being recorded?
A: This example was a part of a biofeedback game that uses EDA and AcqKnowledge‘s Network Data Transfer functionality to provide real-time access to the data for biofeedback. A sphere would materialize after every SCR (skin conductance response). If the goal is for the participant to relax, they try to keep their responses down or else the bar will be tipped over. In another version, the game can be played against a human or computer with the goal of generating more and larger responses than the opponent in order to knock down the bar first. The game adapts the size of the spheres to the max response of the participant so far, thus accounting for individual variability.
- Q: Is the adaptive scaling available in Biopac Student Lab software?
A: Yes.
- Q: Do you use absolute or relative parameters for data error/rejection?A: If you are reviewing the data before the analysis is performed then data quality is a little subjective. During this part of the review, you should be looking at the absolute Skin Conductance Level data, which is the Tonic waveform and make sure that there are no obvious sections of artifact such as sudden jumps or drops in the data. However, the analysis uses specific parameters for rejecting data such as the threshold crossing and the ability to reject any SCR below X% of the maximum SCR response. The default setting for this rejection is 10%, but you can set the value to zero and every SCR will be included in the analysis.
- Q: which is the correct sampling rate for biomedical signals?A: Each physiological signal has its own sample rate which is based on the frequency components within the signal. EDA is a slow signal so you can reliably record the data at 70-100 samples a second to correctly capture the signal. However, if you are looking at facial EMG, the frequency components within the EMG waveform are much higher and you would want to sample that data at >1,000 samples a second.
- Q: What do you use for a calibration signal?A: If you use the module setup function in AcqKnowledge, the system will scale the data for you and you will not require a calibration signal. The system will automatically guide you through this process.
- Q: Is it possible to do deconvolution analysis with AcqKnowledge? What is your opinion of deconvolution-based approaches to measuring SCRs?
A: AcqKnowledge does not currently include an automated deconvolution analysis option, but we are constantly reviewing feature requests. AcqKnowledge can export the data in 3rd-party formats to aid in the process.
EDA Event Markers
- Q: How do you event mark based on performance, e.g., when errors occur or short/ long reaction times on behavioral tasks run on E-Prime?
A: The easiest way it to go into Setup Data Acquisition and select Event Marking. This will allow you to select any Function Key and apply a label to the event mark. In this case, the label could say ‘Error’ and each time that particular Function Key is pressed, an event mark will be inserted with the label “Error” applied to it. The software will allow you to search and jump to any event mark in the file by accessing the Event Palette from the Toolbar. The following Event Marks screencast will provide you with a detailed demonstration of the event mark feature.
- Q: How do you label biometrics data? (i.e. assign a label to a time segment for use in machine learning models)A: The system allows you to enter event marks and segment marks. The segment marks are applied when you start recording a new segment of data when using the Append mode. The event marks can be setup before you start recording data and then as you apply a marker, the marker type and label will be applied to the data. The previous webinar covered the use of events and segment markers. https://www.biopac.com/events/acqknowledge-webinar/
- Q: How can I generate event markers from the network/ other software?
A: With the MP150 system and the STP100C module you can receive data from the parallel port or the StimTracker (and similar device) from any computer. You can send markers over the network to AcqKnowledge using Network Data Transfer (NDT) with NDT licenses. For virtual reality experiments we can provide sample code for how to send markers from Vizard to AcqKnowledge, as well as how to stream physiological data to the virtual reality computer in real-time.
- Q: How do I synch and create markers from my e prime (solving 5 types of math equations)? Right now I have a tech manually mark it.
A: If you use the STP100C and interface between the parallel port on the computer running E-prime, you can send digital markers from E-prime to the MP recording device. The Digital Input to Stim Events analysis option will convert the digital pulses from E-prime into unique events that AcqKnowledge can use for analysis. Click to watch the Digital Input to Stim screencast for additional information about this feature.
- Q: Can I save these preferences for use with other data sets from same study?A: Yes, the Preferences are saved until you change them. If you set the Preferences at the start of the study, every file will be analyzed using the same settings.
- Q: Is there any way to label events after recording by adding an excel file to the recorded stimuli events?A: Yes, you can add labels to any event using the Event Bar. Select the event and type in the label. Unfortunately, there is no automated way to handle this.
- Q: What was the deal with Channels 8-15? Are those the channels you may only use for physiological data collection? I’m a bit confused by that part in the presentation.
A: Digital channels 8-15 can be used to record markers from stimulus presentation software such as E-Prime, Presentation, and Experiment Center.
Temporal Factors and EDA
- Q: I usually have participants sit quietly for at least 5 minutes before beginning EDA recording/data collection. As you would expect, their EDA responses decline during this time. However, I notice that many participants’ EDA responses continue to decline past this period, sometimes for as long as 30 minutes, regardless of what else they might be doing during the study. What can I do to minimize this? I observe this with both MP100 and MP45 systems.
A: The Skin Conductance Level data will typically drop as a subject relaxes and this is quite normal. See A Guide for Analysing Electrodermal Activity (EDA) & Skin Conductance Responses (SCRs) for Psychological Experiments for a good section that covers this particular issue.
- Q: Synchronize between two persons?A: If the data from the two subjects was recorded using the same device, it is possible to use the event marks to synchronize the two subjects. This assumes that the same device is sending the trigger marks to both subjects. If you are not presenting visual stimuli to the subjects, but the data is recoded on the same device, you can assume that the timing between the subjects is good. If you also video the subjects, you can see when they perform certain tasks or answer questions and converse with one another. It is then possible to take measurements around these points and see how the participants are responding. The manual event marking system can also be used to identify certain points of interest during and after the recording. These events can then be used to drive the analysis. Please see the event mark webinar and screencast for further information about event marking. https://www.youtube.com/watch?v=GUEv2GxTCKo&t=940s , https://www.biopac.com/events/acqknowledge-webinar/
- Q: How long should one be seated still to establish a baseline? I often see EDA activity decline over 30 min with subjects at rest.
A: If you are looking at Skin Conductance Level during the baseline, you might see the level drop as the participant relaxes and gets used to the equipment. The important thing is ensure that the subject is relaxed and not engaged in anything during the baseline period. A period of 2-4 minutes should work for a baseline, but I would highly recommend consulting the literature to see what is appropriate for your application.
- Q: Is there a way to input timing files if you do not have a trigger delivery system set up from a stimulus presentation software to BIOPAC?
A: Yes, you can do this with the Scripting software. The following link will provide you with additional information about BIOPAC Basic Scripting. https://www.biopac.com/product/basic-scripting-licenses/
- Q: What is the minimum time between events for event-related EDA analysis?
A: This is a function of latency + rise time + recovery time. We would suggest no less than 8-10 seconds, but this decision is ultimately the researchers. There are also publications on how to deal with analyzing EDA data when events are spaced very closely together; there certainly are some interesting solutions. We recommend that you consult the literature if you have to cut down on the inter-stimulus interval.
- Q: What are Best Practices for measuring long-term EDA?A: I would recommend consulting some of the references listed in the presentation but the following links both cover ambulatory or long-term recordings.
- Q: In analyzing EDA data I’ve been using the following rule with regards to the timing of an event-related SCR – >>Boucsein (1992) suggests that SCRs that start more than 5 sec after the end of a stimulus should be categorized as non-specific<<. Is 5 seconds potentially too much? I’ve had some SCRs that were just under the 0.05 threshold (i.e. 0.045, 0.048). My initial thoughts were to exclude them? I have a reference for the 0.05 threshold but can’t locate it right now. Perhaps this threshold depends on the stimulus/experiment aims/context? Thanks – great presentation
A: Handbook of Psychophysiology. John T. Cacioppo, Gary Berntson, Louis G. Tassinary states 1-3 seconds or 1-4 and I have always used that as my reference. The default setting in AcqKnowledge is 1-4 seconds, but you can change that as I demonstrated in the webinar. The same guide also mentions 0.05 for a threshold for detecting SCRs but is also goes on to say that this threshold was based around the use of legacy paper recording devices. The Society for Psychophysiological Research guideline paper recommends between .01 and .05, based on what the equipment can handle. As I demonstrated in the presentation, the software can reliably detect responses at the .01 level. However, I would definitely look closely at the guidelines and ensure that you are using the correct values.
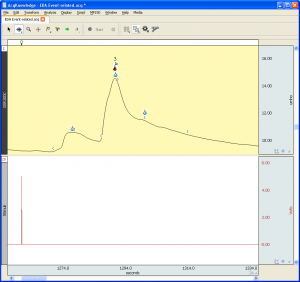
EDA Stimuli plot
Stimulation and EDA
- Q: What type of stimuli/experiment was this last example for?
A: The example which included two digital channels with markers involved auditory stimulation. Two categories of sounds were played to the participant: recordings of screams and recordings of hand claps. They had similar dB levels.
- Q: Are there stimuli every second or so?
A: Such a frequency of stimulation would be too much given the time it takes for responses to reach their peak and decay.
- Q: How I can stimulate the rats?
A: We can deliver a wide range of voltage or current stimulation, including even transcranial current stimulation (tDCS or tACS). It is best to contact us at support@biopac.com since there are many details to consider when designing an experiment with electrical stimulation.
- Q: what if my peristimulus period is less than 5s?A: It will be hard to determine whether a response is specific or non-specific. I would recommend reading the EDA section in the Handbook of Psychophysiology John T. Cacioppo Louis G. Tassinary Gary G. Berntson I’m sure that they will have some recommendations regarding this situation. I am also proving links to some guideline papers that may provide you with further information. https://www.biopac.com/application-note/electrodermal-response-guidelines-gsr100c/
- Q: How can I connect sound stimuli into BIOPAC and sync it with EDA recording?
A: Here are two options for the MP150 System (similar options available for the MP36R):
a) Split the audio output of the computer and feed it directly into the system as an analog channel via the HLT100C and INISO. This would ensure optical isolation.
b) If using stimulus presentation software, send markers over the parallel port to the STP100C module.
There are more solutions available and if you need further information, please contact us at support@biopac.com
Recording EDA in the MRI
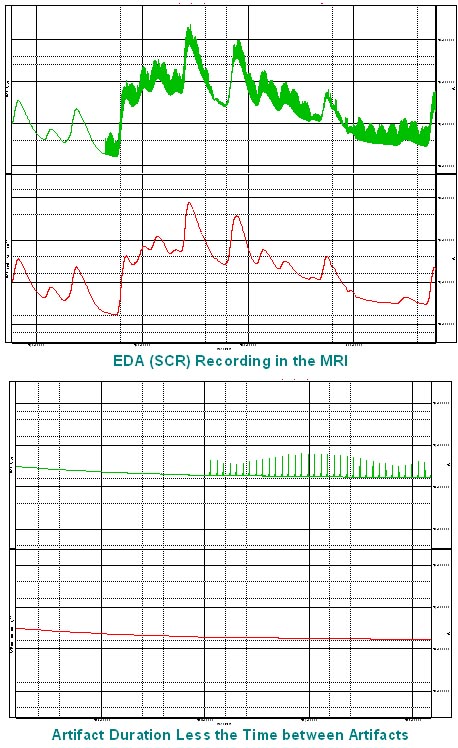
EDA Data recorded in MRI
- Q: Hello, I have a data set of EDA acquired during Magnetic Resonance (MR) acquisitions, and the signal has a lot of noise. Is there an optimal type of filter for EDA measurements collected in this type of environment? or the filter to apply depends on the specific parameters of acquisition within the MR session?A: If you are using the EDA100C-MRI with one of the filtered cable sets MECMRI-TRANS, the data should be pretty good. I would start by employing a 10 Hz Low Pass IIR filter to the data and seeing if that cleans the signal. If you are still suffering from a lot of noise, I would recommend consulting with our support staff because they can review the data and make recommendations. We do have a series of Application Notes that specifically address recording data in the scanner. The following link will provide you with additional information. https://www.biopac.com/application-note/?fwp_app_note_cat=recording-physiological-data-in-mri-or-fmri
- Q: How to compensate for sensitivity to outside (MRI) electromagnetic noise?
A: Make sure that you are using the EDA100C-MRI with one of the BIOPAC MECMRI-TRANS filtered cable sets with suitable electrode leads and electrodes. The filter must be properly attached to the patch panel as per the instructions. The EDA100C-MRI does a nice job of eliminating/reducing gradient noise from the scanner and providing a good EDA signal.
- Q: We are recording in the MRI. Our signal flatlines when the scanner starts and won’t come back even when the scanner stops. Do you have troubleshooting ideas?
A: Make sure that you are using one of our MECMRI-TRANS filtered cable sets with suitable electrode leads and electrodes. The filter should be securely fastened to the patch panel as described in our instructions. If you have everything connected according to our instructions, you should consult with our Support Department.
- Q: How do you prevent interference between GSR and MEG equipment and vice versa?A: We recommend the following setup – EDA100C-MRI amplifier with the MECMRI-TRANS cable and filter with carbon fiber electrode leads and electrodes.
- Q: When calibrating, would it be better to calibrate outside the scanner room or inside?
A: We recommend calibration outside the scanner room.
Non-responders
- Q: In your experience, are non-responders non-responsive from all recording locations? Or would it be useful to try a different recording location if your subject seems to be a non-responder?
A: We have not experimented with this sufficiently. We recommend consulting with the literature.
- Q: I got some negative values for some participants. Could these be non-responders?
A: This is a calibration issue. To fix existing data, contact us at support@biopac.com. For future recordings, make sure you perform the calibration with open circuit (electrodes not connected to the participant) at the start of the recording.
- Q: We also find that some participants have 0 µS (microsiemens) throughout the protocol, despite correct preparation of the skin (including using isotonic gel), and changing electrode placements.
A: In our experience, about 10% of people are non-responders. If in doubt, you can always record some data from yourself when you encounter such a participant. If you use the same setup and same batch of electrodes and get responses yourself, but nothing from the participants, then you may have a non-responder. Also check the location where you are placing the electrodes for callused skin, cuts and abrasions because they will impact the quality of the signal.
Other
- Q: This was fantastic! Keep it coming. Want to make sure that you are fine with us sharing the recordings with our students.
A: By all means, the goal of such events is to share our knowledge with researchers.
- Q: Where can I find interesting articles and reports about BIOPAC and EDA?
A: Visit our website or conduct a Google scholar search for BIOPAC + GSR or BIOPAC + EDA. The search will return over a thousand results.
- Q: Could you comment on medications or other substances/activities (caffeine, alcohol, eating, smoking, medicine and doing sports) that might influence EDA or skin conductance? Do you have any suggestions for accounting for these effects?
A: I would highly recommend performing a literature review for specifics.
- Q: In the BioNomadix good EDA example is the participant moving, what is he/she doing?
A: I believe the participant was seated in a lab environment.
- Q: I actually need help connecting LiveCode with AcqKnowledge. I want to be able to send an event signal from Livecode and have it recorded. I know how to send a signal through the parallel port but I don’t know what the signal should be so that an event signal is marked. I know this isn’t what the webinar is for, but if you have any insight that can be sent to me it would be helpfulA: I am unfamiliar with Livecode, but you can events remotely using BIOPAC Network Data Transfer. This feature will provide you with real-time access to the data and allow you to send AcqKnowledge events from a third party program. The following link will provide you with additional information about NDT. https://www.biopac.com/product/network-data-transfer-licenses/
- Q: Parallel port support PCs is increasingly harder to find. Also presenting stimulus from laptops would be nice. Additionally 64 bit Windows is making software control of parallel ports increasingly difficult. Are you aware of any USB to parallel converter that would work for SENDING EVENT markers into BIOPAC?
A: The STIMTRACKER (STK100) stimulus presentation marker USB interface offers one solution.
Stay Connected