Recording EDA & Other Questions
EDA Questions and Answers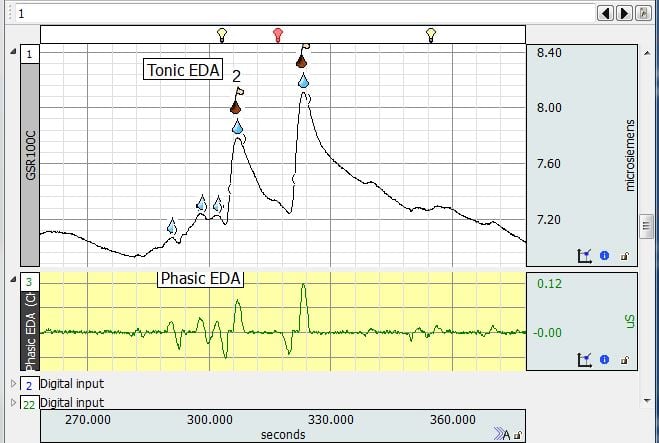
Your colleagues submitted these questions during BIOPAC EDA Webinars.
Topics include
- EDA Recording Systems
- Recording EDA in the MRI
- EDA Markers
- Temporal Factors and EDA
- Stimulation and EDA
- EDA Recording: Non-responders
- Other
Other Sections include
If your question isn’t covered, learn more in EDA: Electrodermal Activity Applications, review EDA Signals & Measurements, or contact BIOPAC Support.
EDA Recording Systems
- Q: Is there an expected difference in reliability of the data between recording EDA in the lab with a MP150 and with a mobile device. We have compared the devices at the same time point and the data doesn’t really look the same.
A: Data recorded from the same electrodes will look identical with one exception: the wireless system uses 12-bit A/D conversion before broadcasting the data, while the wired system uses 16-bit A/D conversion. This means you can detect much smaller changes in skin conductance with the wired system. The minimum resolution with the wireless system is 0.012 µS, while with the EDA100C amplifier it is several orders of magnitude smaller (because you can also change the gain and zero offset of the amplifier). For practical purposes, the resolution of the wireless system will be enough, but this is something to keep in mind if extremely sensitive measurements are necessary.
- Q: What specs (pertaining to validity & reliability) should researchers be aware of when comparing wearable mobile tech devices?
A: One of the best references is to look for publications that have employed the device and technology. BIOPAC products have been cited more than 51,500 times and over 2,630 times specifically for EDA. I would also check how the device is measuring the signal of interest and make sure that the technique is providing you with the data you require for the application. Many wearable devices are great for consumer use but not really suitable for research purposes. The BIOPAC BioNomadix product line has been proven in the lab and is now available for remote subject monitoring.
- Q: Any differences between M35 (previous system with non-disposable EDA electrodes) & my new system (M36) with disposable EDA electrodes?
A: The following link will provide you with information about the MP36R hardware.
- Q: Hi, I was just wondering how the hardware setup changes if you are using the TEL100C telemetry system?
A: Just like with the EDA100C/GSR100C amplifiers, make sure you are in DC mode. You should also make sure the gain you are using is appropriate. Please contact us at support@biopac.com if you have specific questions.
- Q: Can you record several videos simultaneously with AcqKnowledge?
A: Using the CAMSYS4 and CAMSYS8, you can record and synchronize 4 or 8 videos respectively. Furthermore, using the OUT103 led as a sync marker, there is no limit for how many external video cameras you can synchronize with AcqKnowledge and later link the footage to the software. See here. In addition to the multi camera setups there is also a High Frame Rate option for recording fast events.
- Q: Will you be including MP36 systems?
A: The analysis approaches apply equally to data recorded with the MP36 system. The MP36R system comes with the AcqKnowledge software, which was used for the presentation. The BSL software comes with the regular MP36 system (part of our educational BSL package) but has fewer features as it is designed for teaching purposes. AcqKnowledge can open files created with the BSL/MP36 system and, therefore, you can analyze BSL data using the more advanced research software, AcqKnowledge.
- Q: Is it possible to use the BioNomadix system with the MP36 system?
A: It is possible, but please contact us so we can discuss your options.
- Q: Can an EMG headset be used to record wireless EDA data?

BioNomadix Wireless EDA
A: No. EMG equipment records a biopotential voltage while EDA works by passing a current through the body and recording conductance. We have the BN-PPGED module to accomplish wireless EDA recordings.
- Q: Does the BioNomadix wireless logger with PPGED-T transmitter require calibration?
A: The devices are factory calibrated but you can confirm the calibration by using known resistor values. Consult the Support Department for further information.
Recording EDA in the MRI
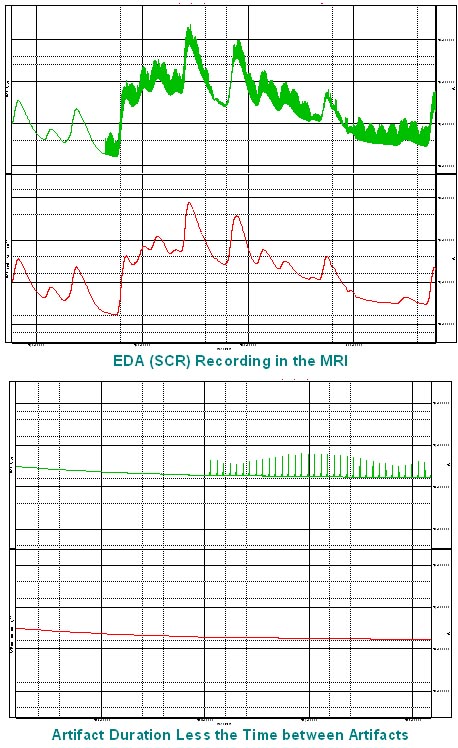
EDA Data recorded in MRI
- Q: Hello, I have a data set of EDA acquired during Magnetic Resonance (MR) acquisitions, and the signal has a lot of noise. Is there an optimal type of filter for EDA measurements collected in this type of environment? or the filter to apply depends on the specific parameters of acquisition within the MR session?A: If you are using the EDA100C-MRI with one of the filtered cable sets MECMRI-TRANS, the data should be pretty good. I would start by employing a 10 Hz Low Pass IIR filter to the data and seeing if that cleans the signal. If you are still suffering from a lot of noise, I would recommend consulting with our support staff because they can review the data and make recommendations. We do have a series of Application Notes that specifically address recording data in the scanner. The following link will provide you with additional information. https://www.biopac.com/application-note/?fwp_app_note_cat=recording-physiological-data-in-mri-or-fmri
- Q: How to compensate for sensitivity to outside (MRI) electromagnetic noise?
A: Make sure that you are using the EDA100C-MRI with one of the BIOPAC MECMRI-TRANS filtered cable sets with suitable electrode leads and electrodes. The filter must be properly attached to the patch panel as per the instructions. The EDA100C-MRI does a nice job of eliminating/reducing gradient noise from the scanner and providing a good EDA signal.
- Q: We are recording in the MRI. Our signal flatlines when the scanner starts and won’t come back even when the scanner stops. Do you have troubleshooting ideas?
A: Make sure that you are using one of our MECMRI-TRANS filtered cable sets with suitable electrode leads and electrodes. The filter should be securely fastened to the patch panel as described in our instructions. If you have everything connected according to our instructions, you should consult with our Support Department.
- Q: How do you prevent interference between GSR and MEG equipment and vice versa?A: We recommend the following setup – EDA100C-MRI amplifier with the MECMRI-TRANS cable and filter with carbon fiber electrode leads and electrodes.
- Q: When calibrating, would it be better to calibrate outside the scanner room or inside?
A: We recommend calibration outside the scanner room.
EDA Event Markers
- Q: How do you event mark based on performance, e.g., when errors occur or short/ long reaction times on behavioral tasks run on E-Prime?
A: The easiest way it to go into Setup Data Acquisition and select Event Marking. This will allow you to select any Function Key and apply a label to the event mark. In this case, the label could say ‘Error’ and each time that particular Function Key is pressed, an event mark will be inserted with the label “Error” applied to it. The software will allow you to search and jump to any event mark in the file by accessing the Event Palette from the Toolbar. The following Event Marks screencast will provide you with a detailed demonstration of the event mark feature.
- Q: How do you label biometrics data? (i.e. assign a label to a time segment for use in machine learning models)A: The system allows you to enter event marks and segment marks. The segment marks are applied when you start recording a new segment of data when using the Append mode. The event marks can be setup before you start recording data and then as you apply a marker, the marker type and label will be applied to the data. The previous webinar covered the use of events and segment markers. https://www.biopac.com/events/acqknowledge-webinar/
- Q: How can I generate event markers from the network/ other software?
A: With the MP150 system and the STP100C module you can receive data from the parallel port or the StimTracker (and similar device) from any computer. You can send markers over the network to AcqKnowledge using Network Data Transfer (NDT) with NDT licenses. For virtual reality experiments we can provide sample code for how to send markers from Vizard to AcqKnowledge, as well as how to stream physiological data to the virtual reality computer in real-time.
- Q: How do I synch and create markers from my e prime (solving 5 types of math equations)? Right now I have a tech manually mark it.
A: If you use the STP100C and interface between the parallel port on the computer running E-prime, you can send digital markers from E-prime to the MP recording device. The Digital Input to Stim Events analysis option will convert the digital pulses from E-prime into unique events that AcqKnowledge can use for analysis. Click to watch the Digital Input to Stim screencast for additional information about this feature.
- Q: Can I save these preferences for use with other data sets from same study?A: Yes, the Preferences are saved until you change them. If you set the Preferences at the start of the study, every file will be analyzed using the same settings.
- Q: Is there any way to label events after recording by adding an excel file to the recorded stimuli events?A: Yes, you can add labels to any event using the Event Bar. Select the event and type in the label. Unfortunately, there is no automated way to handle this.
- Q: What was the deal with Channels 8-15? Are those the channels you may only use for physiological data collection? I’m a bit confused by that part in the presentation.
A: Digital channels 8-15 can be used to record markers from stimulus presentation software such as E-Prime, Presentation, and Experiment Center.
Temporal Factors and EDA
- Q: I usually have participants sit quietly for at least 5 minutes before beginning EDA recording/data collection. As you would expect, their EDA responses decline during this time. However, I notice that many participants’ EDA responses continue to decline past this period, sometimes for as long as 30 minutes, regardless of what else they might be doing during the study. What can I do to minimize this? I observe this with both MP100 and MP45 systems.
A: The Skin Conductance Level data will typically drop as a subject relaxes and this is quite normal. See A Guide for Analysing Electrodermal Activity (EDA) & Skin Conductance Responses (SCRs) for Psychological Experiments for a good section that covers this particular issue.
- Q: Synchronize between two persons?A: If the data from the two subjects was recorded using the same device, it is possible to use the event marks to synchronize the two subjects. This assumes that the same device is sending the trigger marks to both subjects. If you are not presenting visual stimuli to the subjects, but the data is recoded on the same device, you can assume that the timing between the subjects is good. If you also video the subjects, you can see when they perform certain tasks or answer questions and converse with one another. It is then possible to take measurements around these points and see how the participants are responding. The manual event marking system can also be used to identify certain points of interest during and after the recording. These events can then be used to drive the analysis. Please see the event mark webinar and screencast for further information about event marking. https://www.youtube.com/watch?v=GUEv2GxTCKo&t=940s , https://www.biopac.com/events/acqknowledge-webinar/
- Q: How long should one be seated still to establish a baseline? I often see EDA activity decline over 30 min with subjects at rest.
A: If you are looking at Skin Conductance Level during the baseline, you might see the level drop as the participant relaxes and gets used to the equipment. The important thing is ensure that the subject is relaxed and not engaged in anything during the baseline period. A period of 2-4 minutes should work for a baseline, but I would highly recommend consulting the literature to see what is appropriate for your application.
- Q: Is there a way to input timing files if you do not have a trigger delivery system set up from a stimulus presentation software to BIOPAC?
A: Yes, you can do this with the Scripting software. The following link will provide you with additional information about BIOPAC Basic Scripting. https://www.biopac.com/product/basic-scripting-licenses/
- Q: What is the minimum time between events for event-related EDA analysis?
A: This is a function of latency + rise time + recovery time. We would suggest no less than 8-10 seconds, but this decision is ultimately the researchers. There are also publications on how to deal with analyzing EDA data when events are spaced very closely together; there certainly are some interesting solutions. We recommend that you consult the literature if you have to cut down on the inter-stimulus interval.
- Q: What are Best Practices for measuring long-term EDA?A: I would recommend consulting some of the references listed in the presentation but the following links both cover ambulatory or long-term recordings.
- Q: In analyzing EDA data I’ve been using the following rule with regards to the timing of an event-related SCR – >>Boucsein (1992) suggests that SCRs that start more than 5 sec after the end of a stimulus should be categorized as non-specific<<. Is 5 seconds potentially too much? I’ve had some SCRs that were just under the 0.05 threshold (i.e. 0.045, 0.048). My initial thoughts were to exclude them? I have a reference for the 0.05 threshold but can’t locate it right now. Perhaps this threshold depends on the stimulus/experiment aims/context? Thanks – great presentation
A: Handbook of Psychophysiology. John T. Cacioppo, Gary Berntson, Louis G. Tassinary states 1-3 seconds or 1-4 and I have always used that as my reference. The default setting in AcqKnowledge is 1-4 seconds, but you can change that as I demonstrated in the webinar. The same guide also mentions 0.05 for a threshold for detecting SCRs but is also goes on to say that this threshold was based around the use of legacy paper recording devices. The Society for Psychophysiological Research guideline paper recommends between .01 and .05, based on what the equipment can handle. As I demonstrated in the presentation, the software can reliably detect responses at the .01 level. However, I would definitely look closely at the guidelines and ensure that you are using the correct values.
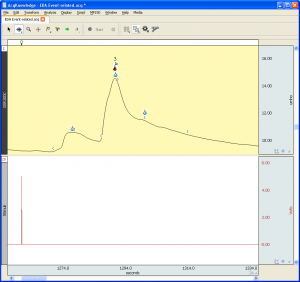
EDA Stimuli plot
Stimulation and EDA
- Q: What type of stimuli/experiment was this last example for?
A: The example which included two digital channels with markers involved auditory stimulation. Two categories of sounds were played to the participant: recordings of screams and recordings of hand claps. They had similar dB levels.
- Q: Are there stimuli every second or so?
A: Such a frequency of stimulation would be too much given the time it takes for responses to reach their peak and decay.
- Q: How I can stimulate the rats?
A: We can deliver a wide range of voltage or current stimulation, including even transcranial current stimulation (tDCS or tACS ). It is best to contact us at support@biopac.com since there are many details to consider when designing an experiment with electrical stimulation.
- Q: what if my peristimulus period is less than 5s?A: It will be hard to determine whether a response is specific or non-specific. I would recommend reading the EDA section in the Handbook of Psychophysiology John T. Cacioppo Louis G. Tassinary Gary G. Berntson I’m sure that they will have some recommendations regarding this situation. I am also proving links to some guideline papers that may provide you with further information. https://www.biopac.com/application-note/electrodermal-response-guidelines-gsr100c/
- Q: How can I connect sound stimuli into BIOPAC and sync it with EDA recording?
A: Here are two options for the MP150 System (similar options available for the MP36R):
a) Split the audio output of the computer and feed it directly into the system as an analog channel via the HLT100C and INISO. This would ensure optical isolation.
b) If using stimulus presentation software, send markers over the parallel port to the STP100C module.
There are more solutions available and if you need further information, please contact us at support@biopac.com
Non-responders
- Q: In your experience, are non-responders non-responsive from all recording locations? Or would it be useful to try a different recording location if your subject seems to be a non-responder?
A: We have not experimented with this sufficiently. We recommend consulting with the literature.
- Q: I got some negative values for some participants. Could these be non-responders?
A: This is a calibration issue. To fix existing data, contact us at support@biopac.com. For future recordings, make sure you perform the calibration with open circuit (electrodes not connected to the participant) at the start of the recording.
- Q: We also find that some participants have 0 µS (microsiemens) throughout the protocol, despite correct preparation of the skin (including using isotonic gel), and changing electrode placements.
A: In our experience, about 10% of people are non-responders. If in doubt, you can always record some data from yourself when you encounter such a participant. If you use the same setup and same batch of electrodes and get responses yourself, but nothing from the participants, then you may have a non-responder. Also check the location where you are placing the electrodes for callused skin, cuts and abrasions because they will impact the quality of the signal.
Other
- Q: This was fantastic! Keep it coming. Want to make sure that you are fine with us sharing the recordings with our students.
A: By all means, the goal of such events is to share our knowledge with researchers.
- Q: Where can I find interesting articles and reports about BIOPAC and EDA?
A: Visit our website or conduct a Google scholar search for BIOPAC + GSR or BIOPAC + EDA. The search will return over a thousand results.
- Q: Could you comment on medications or other substances/activities (caffeine, alcohol, eating, smoking, medicine and doing sports) that might influence EDA or skin conductance? Do you have any suggestions for accounting for these effects?
A: I would highly recommend performing a literature review for specifics.
- Q: In the BioNomadix good EDA example is the participant moving, what is he/she doing?
A: I believe the participant was seated in a lab environment.
- Q: I actually need help connecting LiveCode with AcqKnowledge. I want to be able to send an event signal from Livecode and have it recorded. I know how to send a signal through the parallel port but I don’t know what the signal should be so that an event signal is marked. I know this isn’t what the webinar is for, but if you have any insight that can be sent to me it would be helpfulA: I am unfamiliar with Livecode, but you can events remotely using BIOPAC Network Data Transfer. This feature will provide you with real-time access to the data and allow you to send AcqKnowledge events from a third party program. The following link will provide you with additional information about NDT. https://www.biopac.com/product/network-data-transfer-licenses/
- Q: Parallel port support PCs is increasingly harder to find. Also presenting stimulus from laptops would be nice. Additionally 64 bit Windows is making software control of parallel ports increasingly difficult. Are you aware of any USB to parallel converter that would work for SENDING EVENT markers into BIOPAC?
A: The STIMTRACKER (STK100) stimulus presentation marker USB interface offers one solution.
Stay Connected