EDA Electrode & Recording Preparation
EDA Questions and Answers
Your colleagues submitted these questions during BIOPAC EDA Webinars.
Topics include
- EDA Skin Prep/Gel Application
- EDA Electrode Placement
- Grounding
Other Sections include
If your question isn’t covered, learn more in EDA: Electrodermal Activity Applications, review EDA Signals & Measurements, or contact BIOPAC Support.
EDA Skin Prep/Gel Application
- Q: What is the process to put on the electrodes on the participants to get a good signal? Should I have them wash their hands? Use alcohol? Conductance gel?
A: No alcohol should be used as it dries out the skin. If the participant must wash their hands, use just plain water. The disposable electrodes (EL507 and EL509 for MRI) are already pre-gelled, but if they are dry, GEL101 isotonic gel must be added. When using reusable electrodes, such as the TSD203, fill the cavity with GEL101.
- Q: Isn’t it important to control the area of contact? If gel covers a larger surface area, conductance levels will be higher.
A: With both disposable and reusable electrodes, the surface area remains quite consistent between participants. For example, the glue of the EL507 and EL509 electrodes is quite strong so the gel cannot really spread beyond the contact area of the electrode once you apply it to the skin and the sponge pad helps to keep the gel in place.
- Q: Do we need to apply the gel also on the electrodes for one use? How much? Do we apply it on skin or on electrodes? Thanks.
A: If the electrodes are fresh, you do not have to do anything. If they have dried out (you will know it, as they will feel dry to the touch and may even have a crust) then add gel as per the procedure that was described during the webinar.
- Q: Is there any downside (other than using up GEL101) to “reviving” electrodes that have not yet dried out? In other words, erring on the side of caution.
A: There is a downside. Since you are mixing the fresh gel with the dried gel, you are altering the salinity. Thus, the resulting gel mix will be more likely to saturate the sweat glands. Placing extra gel on the electrodes is a temporary solution until you can obtain new fresh electrodes.
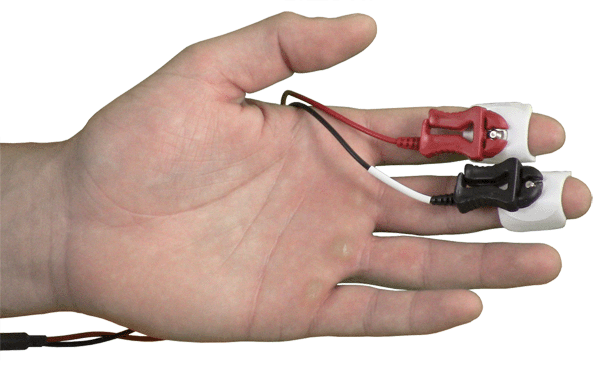
Disposable EDA Setup: SS57LA leads + EL507 EDA electrodes
- Q: How does pressure applied in the electrodes area affect EDA?
A: If you are using the TSD203 EDA transducer or SS3LA reusable electrodes, you want to avoid over tightening the strap because you may occlude the vessels in the finger. The Velcro strap should be just tight enough to hold the electrode in place and prevent it from moving during the recording session. If you are using disposable electrodes EL507, you can also use surgical tape to ensure that the electrodes remain attached to the participant but the tape should not be applied too tightly, just tight enough to hold the electrode in place.
- Q: When a participant’s hand is cold, there is no response or the EDA data looks very strange. What are the recommendations on subject and room temperature settings during data collection with EDA?
A: Subjects with cold hands will not normally provide good EDA data. Room temperature should be ambient between 22-240C—not so cold as to have the subjects feel chilled, not so hot as to have the subjects sweat. Either of these conditions will adversely impact the quality of data.
- Q: How about some participants who have cold hands? Does their temperature of hands influence recording EDA data?
A: Yes, we have observed that cold hands will negatively impact the quality of EDA data, reducing the SCR size in some participants. This subject is also discussed in detail in the Handbook of Psychophysiology.
- Q: Is it just the reusable electrodes that dry out, or do the SS3L electrodes dry out as well?
A: The SS3L EDA transducer contacts are filled with gel at the start of the study, then they are cleaned and stored away. Disposable electrodes are pre-gelled and if they are not stored properly or they are past the expiration date, the gel may be dry.
EDA Electrode Placement
- Q: Electrode placement table—what’s the paper title?
A: Emotional sweating across the body: Comparing 16 different skin conductance measurement locations, Physiology & Behavior 106 (2012) 298–304
- Q: What are the ideal locations for the FPS (startle) electrodes?
A: It is best to refer to the Committee report on Guidelines for human startle eyeblink electromyographic studies.
- Q: What about electrode placement on thenar and hypothenar?
A: The Committee report on Publication recommendations for electrodermal measurements, just like the Handbook of Physiology, refers to this and the volar phalanges placement as recommended. It does not compare the two, however, so I recommend reviewing the literature to see if there is an indication which placement is the best, thenar and hypothenar or volar phalanges. It is hard for us to make a recommendation when there are so many variables.
- Q: Is it possible to use ECG electrodes at the palm of the hand?
A: ECG electrodes should not be used to record EDA because the gel is not isotonic.
- Q: Electrode placement. I have used some of the systems that purport to record from wrist. Doesn’t seem to work.
A: Please see the presentation for recommended and documented areas for electrode placement.
- Q: I’m particularly interested in getting better data from elderly participants.
A: The main issue will be ensuring that you have good electrode contact with the skin. Make sure that you are using fresh electrodes that have plenty of gel and test the subject by asking them to take a breath and hold it. The electrodes should be on the participant for at least 5 minutes and this may take a little longer with some subjects. Poor peripheral circulation will also have an impact on the quality of the signal. The import thing is to test the subjects before starting the recording and making sure that you are getting good responses from them. I would also consult the literature to see if there are specific recommendations for your study population.

Reusable EDA Transducer: TSD203 or SS57L
- Q: What about recording EDA from the underside of toes with the TSD203 electrodes?
A: The instep of the foot and the palmar surface of the hand have the highest concentration of sweat glands on the body and are thus ideal locations to record from.
- Q: When I check the electrode setup with EL-CHECK, I always get the red or orange light as a result—is this normal? If not, what can I do to get a better signal?
A: Impedance checking is not necessary for EDA. Isotonic GEL101 for EDA recording is not as conductive as regular electrode gel, such as GEL100, and values will naturally be lower. If you are asking how to improve the impedance for biopotential recordings, such as EMG, EEG, etc. then the following will help: abrade the skin using ELPAD or place the electrodes 5-10 minutes in advance. For a full list of recommendations, see Tips for Recording Good Data.
EDA Grounding
- Q: When using other measurements with EDA, for instance ECG or EMG, which one should be used with the lead? Does it matter, or is it better to have it on a particular measurement?
A: The subject is grounded by the EDA amplifier. This means that you do not have to add an additional ground on the other biopotential signals – ECG or EMG. However, if you would like to run an additional ground, without creating a possible ground loop, you should use one of the CBL205 adapters. The CBL205 plugs into the ground on the biopotential amplifier and then the ground electrode lead plugs into the other end of the adapter.
- Q: I have a question about safe grounding. We often collect ECG and ground the subject with three lead set up that way. We don’t ground on any of our other channels. But your slide suggested that we need to have a separate filter to place in between the lead and the EDA amplifier for safety. Please elaborate in the Q/A document.
A: When using EDA, you are already grounded via the VIN- connection of the EDA100C/GSR100C amplifier. Thus, no other ground is necessary, not even an ECG ground. However, you can certainly use other grounds, just make sure to use a CBL205 cable at the ground lead connection for any additional grounds you may want to use.
A second ground can be very useful when recording EEG or EMG (because the quality of the ground connection at the finger with isotonic gel is not the best possible and for these sensitive signals it’s nice to have a separate ground) or in the event that the EDA lead gets disconnected through movement artifact, etc.
- Q: What do you recommend if the participant is sweating (and some people sweat more than others) over the course of activity for which we are collecting EDA for?
A: The hands are not a principal site for thermoregulatory sweating so the impact on recorded EDA should be minimal.
- Q: What are the best electrodes to use with DC voltage? Which electrodes do you use with EDA100C? How long AgCl electrodes (if needed) could be used with the DC system? Can you use 3M Red Dot Monitoring electrodes? How should we apply gel on the electrodes?
A: Please review the section on gel application to electrodes. We strongly recommend TSD203 (MP150) or SS3LA (MP36R) reusable electrodes or EL507 disposable electrodes (use only EL509 electrodes for recording in the MRI). These have been extensively documented in multiple papers. We also strongly recommend GEL101, as other gels are not isotonic and will have a salt concentration which may impact data quality. Dry electrodes may be salvaged—see the presentation—but for best results we recommend using fresh electrodes.
- Q: So should a ground electrode be used (in addition to the VIN-) if the EDA100C is the only amplifier being used?
A: No, an additional ground electrode is not required when recording EDA. The subject is automatically grounded through the EDA electrodes. If you want to use an additional ground electrode, use the CBL205 in series with the ground cable of the other amplifier. This is not typically recommended when only recording EDA. Please read this Grounding Notes.
Stay Connected