Exchanging files
The software is backwards compatible, so newer software should not have trouble opening files created by older software. However, as the software is developed, new features are added. Older versions of AcqKnowledge generally cannot read files created by newer versions. Two types of error message are commonly encountered when an older version of AcqKnowledge tries to read a file created by a newer version:
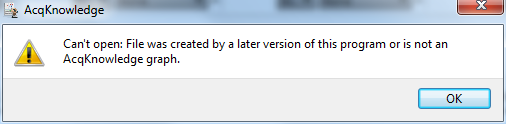
or
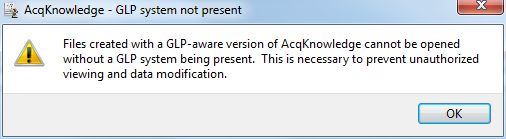
This latter message may also appear when a user attempts to open a file created by Biopac Student Lab but indicates to the software that the file is an AcqKnowledge graph file. When presented with a dialog box such as the following:
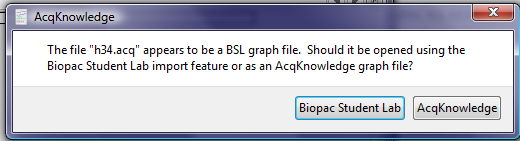
understand that the question is about the format of the file being imported, not the format that you want the file to have after you’ve imported it. If the file was created by Biopac Student Lab and you click the “AcqKnowledge” button, the software will not be able to open the file and will instead generate an error message. The solution in that case is to click “Biopac Student Lab” when opening the file in AcqKnowledge.
If a file cannot be opened because it was created by a newer version, the first step toward a resolution is to determine what the two versions are. Version information is available under “Help > About AcqKnowledge…”
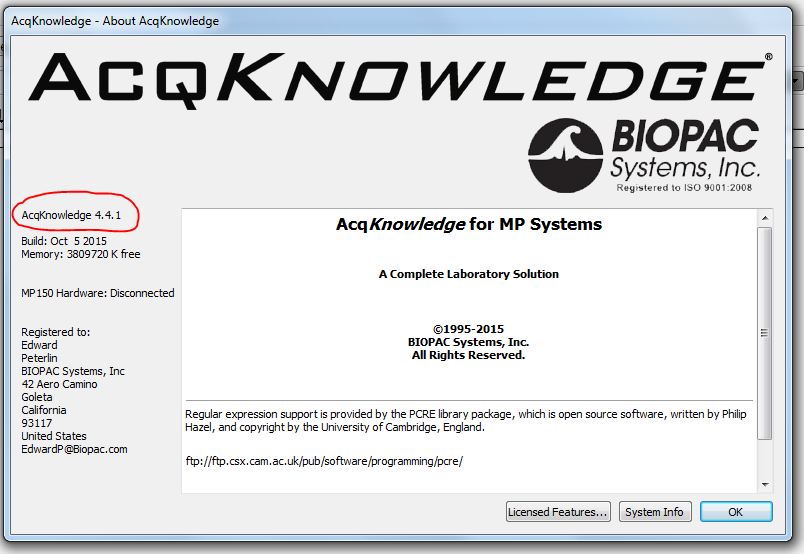
If the two versions differ only in the third number (e.g., the newer version is 4.3.1 and the older version is 4.3.0), contact BIOPAC Support and request an update. If the first or second number is different (e.g., 4.3.1 and 4.4.0), then the owner of the older software license might consider purchasing an upgrade. The alternative may result in a loss of some information:
The sender may save data in an older AcqKnowledge graph file format. Choose “File > Save As…”, and specify that the destination file should be in Windows version 3 format (even if the plan is to open the file on a Mac):
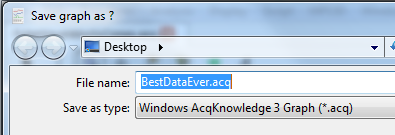
Compared to version four, version three files have limitations in event mark type and placement, and they do not contain any information about the modules used for data collection. Any event marks that were not already either Append events or default global events will be converted to default global events. Meta-data stored as module information via channel setup will be lost in the conversion as will information about edits to the data. Also, journals are capable of containing formatting and other types of information in newer versions, but only plain text in version three. Consequently, the journal in a file saved as version three may not be a faithful copy of the journal in the original file. However, all of the data will be preserved exactly as it was in the newer-formatted file.
Stay Connected