277 – Transcranial Current Stimulation Using STMISOLA
Transcranial Current Stimulation is an electrical stimulation method, which employs a direct current of value typically between 1 and 2 milliamps (ma). This method is typically oriented to neuronal stimulation in the brain. Depending on the type of electrodes used for stimulation, and their associated placement on the scalp and body, it may be called Transcranial Direct Current Stimulation (tDCS), High Definition Transcranial Direct Current Stimulation (HD-tDCS), or Transcranial Alternating Current Stimulation (tACS).
Upon first introduction tDCS/tACS employed the use of large surface area electrodes (25 cm2 – 64 cm2), and electrodes—either carbon composition or metal—were isolated from the skin surface with conductive gel soaked sponges, to help reduce ion transfer from electrodes into the skin and to reduce current density at the electrode sites. tDCS/tACS can also be employed with Ag/AgCl electrode arrays.
- Total arbitrary control of current wave shape is possible (DC or AC stimulation).
- Multiple STMISOLA units can be applied to the same subject.
- Electrode configurations are highly mutable.
- Exact output current applied can be sampled.
- Other physiological signals can be sampled simultaneously to look for tDCS/tACS impact on hemodynamics, sympathetic or parasympathetic nervous system.
This application note illustrates how a HD-tDCS setup can be constructed using the following BIOPAC equipment:
Sample tDCS Hardware and Setup
Set up is identical for tDCS, HD-tDCS, or tACS, except for the fact that tDCS/HD-tDCS is unipolar and tACS is bipolar.
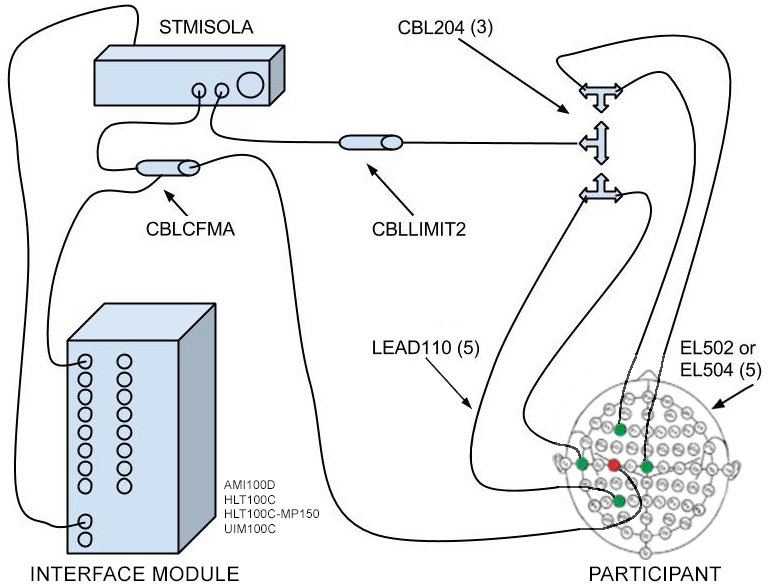 Basic setup for stimulation only:
Basic setup for stimulation only:
1 x MP160/HLT or MP150/UIM (Data acquisition system)
1 x STMISOLA (Isolated linear stimulator)
1 x CBLCFMA (Current feedback monitor)
1 x CBLLIMIT2 (Current Limiter 2 ma)
3 x CBL204 (Y–electrode lead coupler)
5 x LEAD110 (Electrode lead, unshielded)
1 pk. EL502 or EL504 (Solid gel electrodes)
If also recording biopotentals at the same time:
MP160 setup: add 1 x INISO (Analog signal isolator)
MP150 setup: add 1 x HLT100C (High-level transducer amp) + 1 x INISO (Analog signal isolator)
Connection guide: CBLCFMA – INISO – HLT100C
Associated Applications
- Psychophysiology - Record and analyze BP, ECG, HRV, EDA, EMG, EEG, EOG, RSP, etc. Interface to stimulus presentation programs...use automated analysis routines to easily score and analyze data.
Stay Connected