284 – fNIR Systems – Dark Current Measurements
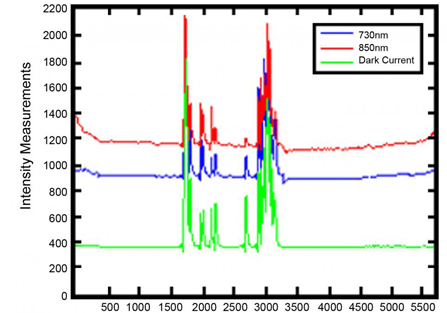 fNIR Systems extract measurements from an LED light source at wavelengths of 730 nm and 850 nm, and also provide a unique feature that can be useful for identifying certain types of noise within recordings. This feature enables the fNIR imager to record measurements under “dark current” conditions.
fNIR Systems extract measurements from an LED light source at wavelengths of 730 nm and 850 nm, and also provide a unique feature that can be useful for identifying certain types of noise within recordings. This feature enables the fNIR imager to record measurements under “dark current” conditions.
Dark current measurements are always recorded in fNIR data output files (.nir and .oxy) and can be monitored during sensor placement, baseline, and overall testing measurement periods by selecting **** under **** buttons in COBI studio.
Time periods during which dark current measurements do not remain flat in close correlation with measurements taken at 730 nm and 850 nm can help identify noisy data segments. These regions can subsequently be marked and discarded from further hemoglobin content extraction analysis.
Noise is not always as obvious the sample shown, and dark current measurements offer a justifiable secondary measure for verifying the presence of noise in all cases.
Associated Applications
- fNIRS Functional Near Infrared Optical Brain Imaging - Measure and analyze oxygen level changes in the prefrontal cortex of human subjects during cognitive tasks or stimulation. Sensors available for adult or pediatric applications.
Stay Connected